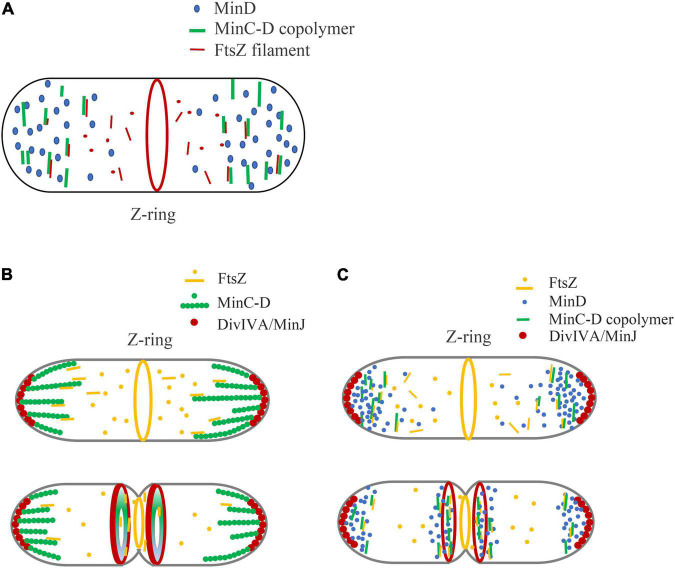
FIGURE 4.
A new model is proposed to explain the regulation of Min system on Z-ring assembly positioning, emphasizing the role of MinC-D copolymer. (A) In general, we think that the binding of MinC-D copolymer to FtsZ protofilament is the key to Z-ring localization regulation. MinC will coassemble with MinD in the area of high concentration of MinD. The tight binding between MinC-D copolymers and FtsZ filaments not only greatly enhances the binding coefficient between MinC and FtsZ subunits, but also can capture FtsZ protofilaments, and prevent their diffusion to the ends of the cell. This may occur in both MinCDE and MinCDJ systems. (B,C) Show our new model of how Min proteins regulate Z-ring position in B. subtilis, if Min proteins form stable structures (B) or dynamic structures (C). Whether MinC/MinD/DivIVA/MinJ complex is stable or dynamic in vivo is controversial. The location of DivIVA determines the uneven distribution of MinD proteins: at the early stage of division, MinD is only located at both ends of the cell, and when membrane invagination begins, MinD accumulates both at the cell poles and midcell. MinC-D copolymers are formed in the area of high concentration of MinD, and form a relatively stable complex (B) or dynamic complex (C) with DivIVA and MinJ. If the complex is dynamic, MinC-D copolymers can be released from DivIVA and accumulated near DivIVA/MinJ complex. This protein complex, when located at the ends of the cell, prevents FtsZ from diffusing to the ends of the cell. And when it is in the middle, this complex can further restrict the Z-ring to a narrow area in the middle. After the MinC-D copolymers and FtsZ protofilaments are tightly combined, MinC accelerates the depolymerization of FtsZ protofilaments.