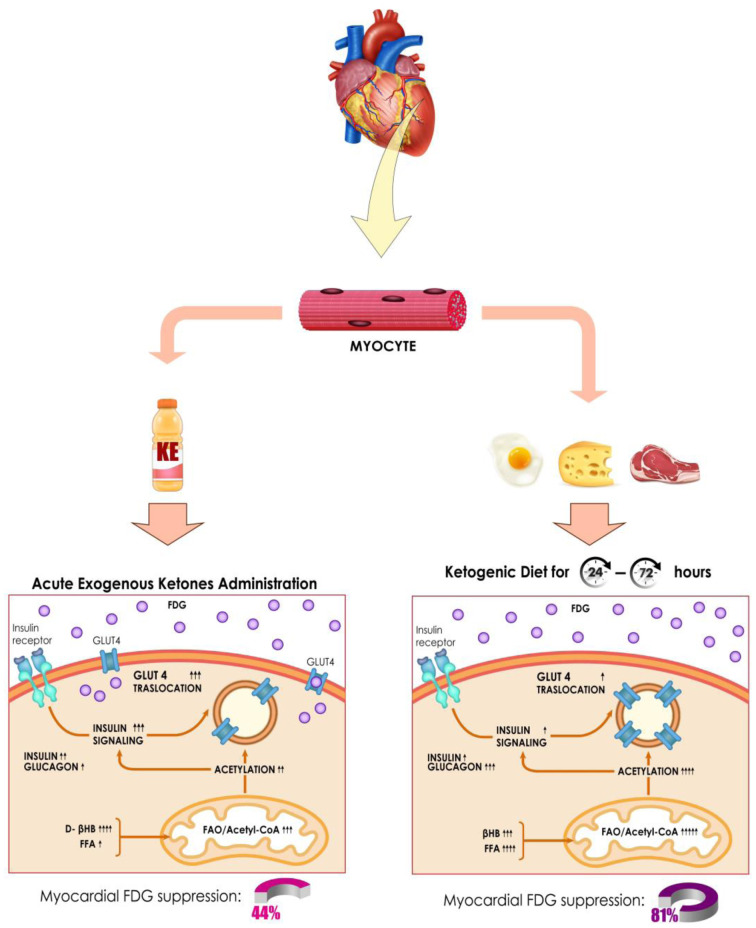
FIGURE 7.
Proposed molecular mechanisms associated with myocardial 18F-FDG suppression between acute exogenous ketone administration (lower left) and endogenous ketosis induced by dietary modification (lower right). Posttranslational protein acetylation and attenuation of insulin signaling are both required mechanisms for successful inhibition of glucose transporter member 4 (GLUT4) translocation. Acute administration of KE drink leads to a severalfold increment in β-hydroxybutyrate (βHB) levels but also appears to decrease free fatty acid (FFA) levels, which may ultimately affect modulation of insulin signaling and process of acetylation. In contrast, although KD yields lower βHB levels after 24–72 h, it increases FFA and glucagon and lowers insulin levels on a sustained fashion, eventually augmenting fatty acid oxidation (FAO) over glucose oxidation (“Randle effect”), increasing protein acetylation, and limiting insulin signaling. These molecular differences in substrate handling may explain why myocardial 18F-FDG suppression rates were 44% for KE and 81% for KD. Adapted from “Insulin Mechanism,” by BioRender.com (2021 (17)).