Abstract
The main reservoir hosts of nematodes of the genus Trichinella are wild carnivores, although most human infections are caused by the consumption of pork. This group of zoonotic parasites completes the entire natural life cycle within the host organism. However, there is an important phase of the cycle that has only been highlighted in recent years and which concerns the permanence of the infecting larvae in the striated muscles of the host carcasses waiting to be ingested by a new host. To survive in this unique biological niche, Trichinella spp. larvae have developed an anaerobic metabolism for their survival in rotting carcasses and, for some species, a resistance to freezing for months or years in cold regions. Climate changes with increasingly temperatures and reduction of environmental humidity lower the survival time of larvae in host carcasses. In addition, environmental changes affect the biology and ecology of the main host species, reducing their number and age composition due to natural habitat fragmentation caused by increasing human settlements, extensive monocultures, increasing number of food animals, and reduction of trophic chains and biodiversity. All of these factors lead to a reduction in biological and environmental complexity that is the key to the natural host-parasite balance. In conclusion, Trichinella nematodes can be considered as an indicator of a health natural ecosystem.
Keywords: Globalization, Climate change, Trichinella, Epidemiology, Human, Zoonosis
Highlights
-
•
Globalization and climate change negatively affect the Trichinella-host relationship.
-
•
Changes of animal behavior and human population growth disturb the Trichinella biology.
-
•
There is a strong relationship between Trichinella spp. prevalence and habitat wildness.
-
•
High environmental temperature reduces the Trichinella larva survival in carrions.
-
•
Trichinella nematodes can be considered as an indicator of a natural wild ecosystem.
1. Introduction
Parasites of the genus Trichinella (Class Enoplea, subclass Dorylaimia, order Trichinellida, family Trichinellidae) are peculiar nematodes in which the infecting larva is the first stage larva (L1) unlike the other nematodes whose infecting stage is the third stage larva (L3) (Takahashi, 2021). Today, the taxonomy groups the genus into two major clades based upon the presence or absence of a collagen capsule that surrounds the larvae while in the muscle cell (i.e. the nurse cell). Taxa of the encapsulated clade, which infect only mammals, are T. spiralis, T. nativa, T britovi, T. murrelli, T. nelsoni, T. patagoniensis, T. chanchalensis and 3 genotypes arbitrarily designated Trichinella T6, T8 and T9 with unresolved taxonomies (Zarlenga et al., 2020). The non-encapsulated clade consists of T. pseudospiralis infecting mammals and avian hosts, and T. papuae and T. zimbabwensis infecting mammals and reptiles (Pozio and Zarlenga, 2013). These 13 taxa have a rather simple but unique life cycle, which is perpetuated through a single host i.e. an infected animal is both the intermediate host, where the infective larvae reside, and the definitive host, where sexual maturity and reproduction take place (Takahashi, 2021). However, the epidemiology of these zoonotic parasites is much more complex as biotic and abiotic factors increasingly influenced by human activities, come into play. The aim of this study was to review the factors deriving from globalization and climate change that can impact the epidemiology and natural cycle of nematodes of the genus Trichinella.
2. Globalization and climate change negatively affect the host-parasite ecosystem
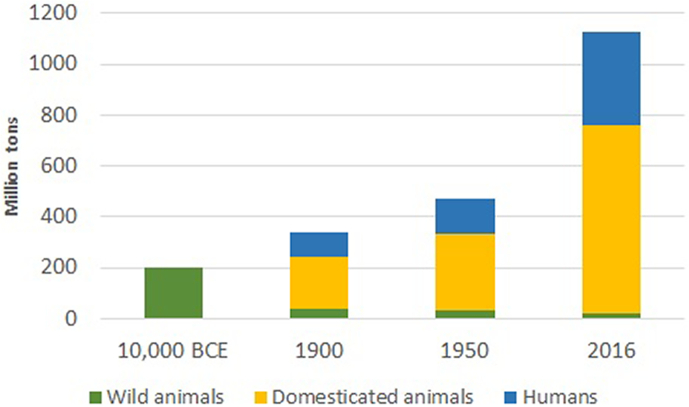
Globalization and climate change cause a reduction of animal species and habitat diversity with a consequent reduction of the biomass of wild animals and an increase in that of domesticated animals (Fig. 1). Wild carnivorous animals are the main natural reservoir hosts of Trichinella sp. nematodes and, unlike most domesticated animal nematodes, the Trichinella biomass is greater in wild than in domesticated animals. It follows that a reduction of wild animal populations and of carnivorous animals in particular, could result in a decline of Trichinella biomass.
Fig. 1.
Changes of terrestrial vertebrate biomass in the last 12,000 years according to Smil (2011).
3. The strong link between parasites and human behaviors
The study of the natural history of parasites (both protozoa and helminths) shows the direct and indirect influence of human behaviors on a great number of these pathogens. For example, Trypanosoma cruzi has spread in the human settlements of South America by its vectors, the Triatominae bugs, which have found an ideal habitat in the houses of rural dwellings with cracked adobe or mud walls (Sandoval et al., 2000). Most foodborne parasites (e.g., Fasciola spp., Echinococcus spp., Taenia spp., Ascaris spp., Trichostrongylus spp., Trichinella spp.) are linked with unsanitary farming practices and/or to wildlife (Jourdan et al., 2018; Pozio, 2018; Weka et al., 2019; Borhani et al., 2021; Dermauw et al., 2021). Unsanitary farming practices are associated with farmers who do not understand the risks and therefore favor parasite transmission (Pozio, 2014). The spread of Trichinella spiralis in most continents is linked to humans who imported infected pigs from Europe to North and South America and New Zealand (Pozio and Zarlenga, 2013). The domestic cycles of Trichinella spp., mainly T. spiralis, exist largely due to human activities. Most T. spiralis infections in horses are caused by humans who feed them with fodder contaminated with infected rodent carcasses, or offal or meat from pigs and game (Pozio, 2014). Trichinella papuae infections in farmed crocodiles are also caused by humans feeding these reptiles with carcasses of slaughtered infected animals such as crocodiles and hunted wild pigs (Pozio et al., 2005; Owen et al., 2014). Trichinella-infected carcasses or the scraps and offal of wild animals left by hunters in the field or killed by cars, represent a great biomass of these parasites that re-enter the wildlife cycle or infect domesticated hosts.
4. Introduction of non-native host species in Europe
In the last century, 44 non-native mammalian species reached Europe, including several carnivores, such as the American mink (Neogale vison), the raccoon (Procyon lotor), the raccoon dog (Nyctereutes procyonoides), and the golden jackal (Canis aureus), which are Trichinella reservoir hosts (Drygala et al., 2008; Arnold et al., 2011; Jernelöv, 2017). The American mink was introduced to fur farms in Northern Europe in the late 1920s and 1930s, and escapees established a feral population that now stretches from the Nordic countries to Russia, Central, Western and Southern Europe (Spain and Italy). Trichinella pseudospiralis has been detected in an American mink of the Bornholm Island of Denmark (Pozio, 2016a). T. pseudospiralis has been also detected in a raccoon of Caucasus whose population originated from North America as a result of escapes and deliberate introductions in the mid-20th century (Garkavi, 1972). From 1928 to 1958, raccoon dogs were introduced in European Russia from the far east, after which this mammal colonized northern and central Europe and some areas of Italy and Spain. All four Trichinella species circulating in Europe (T. spiralis, T. nativa, T. britovi and T. pseudospiralis) have been detected with high prevalence in this carnivore species, which can be considered one of the largest Trichinella reservoir hosts in northern central Europe today (Deksne et al., 2016; Oksanen et al., 2018). The golden jackal though mostly found in southeastern Europe has increased its range to encompass parts of the Baltics in northeastern Europe, Italy, Western Europe (France, Switzerland, Germany), and several other countries. Both Trichinella britovi and T. spiralis have been detected in this reservoir host (Blaga et al., 2008; Széll et al., 2013; Dmitric et al., 2017; Frey et al., 2022).
5. Changes of animal behavior and human population growth
Increased availability of food resources for wild animals in urban and rural areas (Hansen et al., 2020) reduces predation and scavenger behavior, i.e. the main ways of Trichinella transmission (Pozio, 2021). The increase of urban, residential outskirts and suburban areas reduces the wild habitat and the populations of carnivores that settle in these highly anthropized environments (Pozio, 2020); furthermore, these carnivore populations are represented by a large percentage of young individuals and therefore have a low probability of being infected with Trichinella spp. (Bateman and Fleming, 2012). Large carnivore populations such as wolves (Canis lupus), lynxes (Lynx lynx), wolverines (Gulo gulo) and bears (Ursus arctos) are making a comeback in Europe as a consequence of wild herbivores and wild boar population growth and increased availability of food of anthropogenic origin (European Commission, 2019; Pozio, 2020). However, the correlation between the increase in these carnivore populations and the increase in Trichinella biomass cannot be established as a large percentage of these animals feed on food of human origin (e.g., domesticated animals, waste) (Manlick and Pauli, 2020).
6. Relationship between meat trade, globalization and food habits
Pig farms under controlled housing conditions are less susceptible to Trichinella sp. infections (Pozio, 2014; Pozio et al., 2021). In the last 25 years, there were 12 outbreaks of human trichinellosis from pig meat and 5 outbreaks from wild boar meat illegally imported from Eastern to Western Europe. Trichinella spp. imported with horse meat by international trading was the source of 8 outbreaks (2296 cases) of trichinellosis in France and 7 outbreaks (1038 cases) of trichinellosis in Italy. Meat of 12 horses imported to France and Italy from Eastern Europe and North America tested positive for Trichinella spp. (Pozio, 2015). An outbreak of trichinellosis occurred in Belgium from the consumption of wild boar meat imported from Spain (Messiaen et al., 2016), where the last reported infections in humans after eating indigenous wild boar meat had occurred 35 years earlier (Famerée et al., 1979). Since nematodes of the genus Trichinella are mainly circulating among wildlife and backyard or free ranging pigs, these pathogens do not represent a great concern for the international meat trade. From the 1950s to today, according to the literature, there are only 43 reports describing the importation of Trichinella spp. infected animals or meat by international trade to Europe. Most (60%) of these reports refers to live horses or their meat, 18.6% to pigs, 4.7% to wild boars and 14.3% to bears (Pozio, 2015). In contrast, according to the scientific literature, there are many reports of meat from pigs, wild boar and bears, illegally introduced in personal baggage causing trichinellosis outbreaks in several European countries (Pozio, 2015; Rostami et al., 2017).
7. Environmental changes and anthropization
Urban areas are significantly warmer than surrounding rural areas due to human activities, particularly at night, which is called “urban heat island” factor (Theeuwes et al., 2017). Observed precipitation trends are characterized by high variability in space and in time, but climate models clearly indicate a trend towards reduced rainfall in coming decades (Blunden and Arndt, 2020). The combination of reduced rainfall and warmer temperatures generates strong trends towards drier conditions. Frequency and intensity of droughts have already increased significantly and by an increase of water demand related to a strong increase of the human population. Climate change implies significant risks for ecosystems. In addition to direct consequences of climate change, there are many combined consequences of different environmental changes resulting from human pressures, like pollution of air, water and soils, and degradation of land. Land ecosystems are impacted not only by direct consequences of climate change (warming, drought), but also by changes in land use (including abandonment of pasture and extensive crop activities, especially monocultures, in some remote areas and mountains) and urbanization, which provokes landscape fragmentation (European Environment Agency, 2011). Land ecosystems are also impacted by pollution, unsustainable tourism, overexploitation of resources, overgrazing and forest fires. Fire risk increases due to drought and heat waves but also due to changes in land management, bringing longer fire seasons, and potentially more frequent large, severe fires. Fires are generally the result of fuel accumulation during the wet season and increased droughts during the dry season. The megafires triggered by extreme climate events, especially heat wave events, have caused the most extensive burned areas (Huber, 2018). In such scenarios, there is a rapid relocation of fleeing animals and a scarcity of food resulting in the loss of environmental complexity, which is the key to a host-parasite balance. These habitat and climate alterations will have consequences on the main route of transmission of Trichinella larvae present in wild animal carcasses due to the reduction of their survival time resulting from temperature increases and a reduction in humidity.
8. The environmental impact of food animals
Production of food animals has different impacts on the environment due to several factors, the main of which are increased production of CO2 and water consumption (Poore and Nemecek, 2018). Today, pork is the most widely consumed meat, representing 40% of the total worldwide, followed by chicken (29%), beef (24%), sheep and goat (5%) and turkey (2%). Production of one kg of beef, results in the production of 27 kg of CO2 and the consumption of 16,000 l of water; for one kg of pork, 12 kg of CO2 and 6000 l of water; and for one kg of chicken meat, 7 kg of CO2 and 1000 l of water (Gerbens-Leenes et al., 2013). In the future, the production of pork and poultry meat will increase while that of beef will decrease as a percentage of consumed meat. Consequently, it will be necessary to control the farming methods to prevent that, an increase in production, may cause an increase of zoonotic pathogens including Trichinella spp. for which the pig represented in the past and continues to represent even today the main source of infection for humans (Murrell and Pozio, 2011).
9. Climate change and Trichinella
Climate change, besides influencing the behavior of Trichinella sp. host animals, can strongly impact the survival of Trichinella sp. larvae in decaying muscle tissues. The greater the persistence of larval viability in host carrion, the higher the probability of being ingested by a scavenging host. Increased humidity favors the survival of larvae, whereas increased temperatures, increasing periods of drought, and ozone depletion reduce the survival of larvae (Pozio, 2016b). As above reported, climate change increases the risk of hot, dry weather that is likely to fuel wildfires. There is no study on the impact of forest fires on Trichinella epidemiology. However, some information can be drawn from studies on the effects of wildfires on carnivore populations. According to Furnas et al. (2021), carnivores would benefit from landscapes managed for greater, but not maximal, fire severity.
10. The parasites' adaptation to the environment
In spite of the larva-induced angiogenic process that develops around the nurse cell after larval penetration of the muscle cell, larval metabolism is basically anaerobic (Despommier, 1990), which favors its survival in decaying tissues. In fact, Trichinella spp. of the encapsulated clade are dispersed in a way analogous to many nematodes which have hardy eggs or a free-living stage in water or soil. Trichinella spp. have a similar free-living stage, with populations of larvae in the collagen capsules in a special biotope, the carcass, which may even rot. These populations are maintained in a way analogous to helminth egg populations with new carcasses becoming available all the time (Pozio, 2016b). The concept of a free-living stage in the carcass is of high epidemiological significance, since it reduces the importance of predation. Of course, it does not mean that transmission cannot occur via predation, but on a broad scale it is of less epidemiological importance. The persistence of larvae in putrefying flesh is also determined by the environment: high humidity and low temperatures favor survival even when the muscle tissue is completely liquefied. This condition has been proposed as the environment of the “free-living” stage (Madsen, 1974).
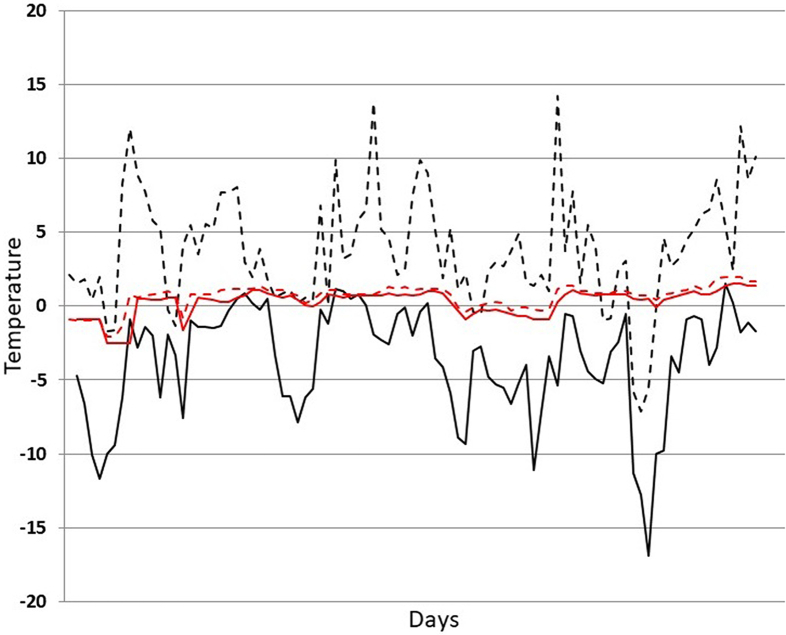
In the natural cycle of the parasite, the importance of larval survivability in animal carcasses is further proved by the resistance of muscle larvae in frozen muscles up to one (T. britovi) or more years (T. nativa, T. chanchalensis and Trichinella T6) (Pozio, 2021). Survival is greatest at temperatures between 0 °C and 20 °C. For example, Trichinella T6 larvae in carnivore muscles frozen at −6.5° to −20 °C survived for at least 4 months (Worley et al., 1986), whereas, Trichinella T6 larvae in carnivore muscles frozen at −20° to −30 °C for 30 days, did not survive (Worley et al., 1990). At lower temperatures, the survival time is reduced, suggesting that the optimal temperature range for survival at freezing temperatures corresponds to the temperature under the snow. This habitat has been named as the “subnivium”. The “subnivium” can be described as “below the snow” because it provides environmental stability (Keppel and Wardell-Johnson, 2012; Pauli et al., 2013). The warmer and more stable conditions within the subnivium are principally driven by snow duration, density, and depth. Trichinella britovi larvae survive longer in carcasses beneath the snow than in those above the snow (Fig. 2). The stability of the environment beneath the snow favors the survival of T. britovi larvae in host muscles, increasing the probability of their transmission to other hosts. The environment above the snow, characterized by sudden temperature variations, causes strong environmental stress for larvae in host carrions causing their death (Rossi et al., 2019). Future climate change scenarios predict warmer winter temperatures, which are often accompanied by an increase in precipitation falling as rain rather than snow and an overall increase in air temperature variability. Disturbances to the subnivium in either extent, duration, or thermal stability can therefore disrupt the regimes that currently provide fitness benefits to a variety of organisms, including cold adapted representatives of the genus Trichinella, resulting in phenological mismatches and enhanced mortality. In Latvia, a correlation between the incidence of T. britovi in wild boar and the number of snow cover days during the period 1976–1993, was observed. The higher the number of snow cover days, the higher the T. britovi incidence in wild boar and vice versa (Kirjušina et al., 2015). In the last 30 years, there has been a strong reduction in the prevalence of T. britovi in the fox populations of the Alps. In 1959 on the Italian side of the Alps, the prevalence ranged from 12% to 58% (Marazza, 1960). In the 1990s, the prevalence had dropped to 5–10% depending on the Alpine valleys. In recent years, the prevalence is 0.0% -0.1% in the same valleys. The reduction of Trichinella sp. infection in red foxes has been also observed in Switzerland, i.e. on the northern side of the Alps. Between 1968 and 1985, the Trichinella sp. prevalence in foxes dropped from 14% to 4% and it was further reduced to 1.6% in 2006–2007 (Frey et al., 2009). What are the possible causes of the marked reduction of T. britovi in the Alpine fox? Three factors may have strongly contributed to drastically reducing the biomass of T. britovi in the fox populations of the Alps: 1. the reduction of snow cover days and depth on the Alps; 2. strong reduction of fox carcasses on the field due to lower hunting pressure due to the decreased commercial value of furs; and 3. increased availability of food resources of human origin.
Fig. 2.
Temperature above and beneath the snow at 1200 m above sea level on the Alps during the winter 2018–2019 (December–April). Dotted black line, daily max temperature above the snow; black line, daily minimum temperature above the snow; dotted red line, daily max temperature beneath the snow; red line, daily minimum temperature beneath the snow. Modified from Rossi et al., 2019. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
11. Conclusions
Animals require a certain amount of habitat to persist and thrive, and habitat loss is one of the most critical drivers of declines in global biodiversity, and results in part from human pressure which reduces the available habitat, particularly for large carnivores, i.e. the most important reservoir hosts of Trichinella spp. Furthermore, natural and anthropogenic environmental changes could affect the structure of animal populations. Different mechanisms such as population fragmentation and size reduction, gene flow interruption and genetic drift can be some of the causes for this. Thus, it can be assumed that habitat disturbance of wild ecosystems and food webs can have considerable long-term effects on the demographic viability of parasite populations due to a reduction or changes of the structure of host populations; this includes an increase in the percentage of young animals and a reduction of the percentage of older animals that are the most important reservoirs of Trichinella spp. The extent to which a species could be affected by habitat disturbance is determined by its degree of specialization and dispersal potential. Two of the main factors influencing the genetic variability of a population are the amount of gene flow between populations and the population size. Anthropogenic changes such as habitat disturbance provoke the reduction of host population size and, in turn, this would mean a reduction of the Trichinella sp. biomass. Given that Trichinella nematodes are strongly linked with carnivores, an ecosystem rich in these hosts should also be the one with a high Trichinella sp. biomass. It follows that a healthy wild ecosystem is one with a high level of infection by Trichinella sp. worms. This is evident in Europe, where the prevalence of Trichinella spp. in wild carnivores is directly proportional to the forest cover of countries and inversely proportional to the density of the human population per square km in these countries (Table 1). In addition, climate change causes a strong perturbation of both the Trichinella sp. host biology and the parasite biology. Although Trichinella larvae spend most of the life cycle (from months up to several years) in the entozoic habitat (larvae in striated muscles) of the host, they are under the influence of environmental temperature and humidity in host carrion. The increase in environmental temperatures, the reduction of the snow cover days and snow depth, and the increase of climatic instability, will reduce the transmission of Trichinella sp. larvae by scavenging animals in the wild habitat. On the contrary, the domestic cycle favored by the incorrect human activity to feed swine with infected pork and game scraps will continue to persist and probably to increase due to the impoverishment of the human population.
Table 1.
Relationship among human population density, percentage of country surface covered by forests and prevalence of Trichinella spp. in wild carnivores in some representative countries of Europe.
| Country | Human density for km2 | Percentage of wild areas | Prevalence of Trichinella spp. in wild carnivores | References |
|---|---|---|---|---|
| Finland | 16 | 78 | 34.7 | Oksanen et al., 2018 |
| Estonia | 28 | 45 | 48–66 | Kärssin et al., 2017, 2021 |
| Latvia | 30 | 54 | 49 | Deksne et al., 2016 |
| Spain | 94 | 36 | 0.3–6.2 | López-Olvera et al., 2011 |
| France | 116 | 30 | 2.7 | Aoun et al., 2012 |
| Poland | 123 | 30 | 4 | Bilska-Zając et al., 2020 |
| Italy | 201 | 30 | 0.4–4.9 | Badagliacca et al., 2016; EFSA, 2021 |
| Germany | 232 | 30 | 0–1.9 | Mayer-Scholl et al., 2016; EFSA, 2021 |
The re-emergence of Trichinella sp. infections in pigs and human trichinellosis was connected with the changes in the social, political and economic systems in Bulgaria, Romania and Argentina during the last years of the 20th century (Cuperlovic et al., 2005; Ribicich et al., 2005). Trichinellosis in humans and Trichinella sp. infections in domesticated pigs increased dramatically during and after the Serbian-Croatian war which occurred from 1991 to 1995 (Cuperlovic et al., 2001; Marinculić et al., 2001). These examples suggest a closely monitoring is needed for events in Ukraine in the coming years due to the disastrous war that has just begun as I finished writing this review.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aoun O., Lacour S.A., Levieuge A., Marié J.L., Vallée I., Davoust B. Screening for Trichinella britovi infection in red fox (Vulpes vulpes) and wild boar (Sus scrofa) in southeastern France. J. Wildl. Dis. 2012;48:223–225. doi: 10.7589/0090-3558-48.1.223. [DOI] [PubMed] [Google Scholar]
- Arnold J., Humer A., Heltai M., Murariu D., Spassov N., Hacklander K. Current status and distribution of golden jackal Canis aureus in Europe. Mammal Rev. 2011;42:1–11. [Google Scholar]
- Badagliacca P., Di Sabatino D., Salucci S., Romeo G., Cipriani M., Sulli N., et al. The role of the wolf in endemic sylvatic Trichinella britovi infection in the Abruzzi region of Central Italy. Vet. Parasitol. 2016;231:124–127. doi: 10.1016/j.vetpar.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Bateman P.W., Fleming P.A. Big city life: carnivores in urban environments. J. Zool. 2012;287:1–23. [Google Scholar]
- Bilska-Zając E., Różycki M., Grądziel-Krukowska K., Bełcik A., Mizak I., Karamon J., et al. Diversity of Trichinella species in relation to the host species and geographical location. Vet. Parasitol. 2020;279 doi: 10.1016/j.vetpar.2020.109052. [DOI] [PubMed] [Google Scholar]
- Blaga R., Gherman C., Seucom D., Cozma V., Boireau P. First identification of Trichinella sp. in golden jackal (Canis aureus) in Romania. J. Wildl. Dis. 2008;44:457–459. doi: 10.7589/0090-3558-44.2.457. [DOI] [PubMed] [Google Scholar]
- Blunden J., Arndt D.S. State of the climate in 2019. B. Am. Meteorol. Soc. 2020;101:S1–S429. [Google Scholar]
- Borhani M., Fathi S., Darabi E., Jalousian F., Simsek S., Ahmed H., et al. Echinococcoses in Iran, Turkey, and Pakistan: old diseases in the new millennium. Clin. Microbiol. Rev. 2021;34 doi: 10.1128/CMR.00290-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperlovic K., Djordjevic M., Pavlovic S., Sofronic-Milasovljevic L. Present status of trichinellosis in Yugoslavia: Serbia. Parasite. 2001;8(S2):S95–S97. doi: 10.1051/parasite/200108s2095. [DOI] [PubMed] [Google Scholar]
- Cuperlovic K., Djordjevic M., Pavlovic S. Re-emergence of trichinellosis in southeastern Europe due to political and economic changes. Vet. Parasitol. 2005;132:159–166. doi: 10.1016/j.vetpar.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Deksne G., Segliņa Z., Jahundoviča I., Esīte Z., Bakasejevs E., Bagrade G., et al. High prevalence of Trichinella spp. in sylvatic carnivore mammals of Latvia. Vet. Parasitol. 2016;231:118–123. doi: 10.1016/j.vetpar.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Dermauw V., Muchai J., Al Kappany Y., Fajardo Castaneda A.L., Dorny P. Human fascioliasis in Africa: a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0261166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despommier D.D. Trichinella spiralis: the worm that would be virus. Parasitol. Today. 1990;6:193–196. doi: 10.1016/0169-4758(90)90355-8. [DOI] [PubMed] [Google Scholar]
- Dmitric M., Vidanovic D., Vaskovic N., Matovic K., Sekler M., Debeljak Z., et al. Trichinella infections in red foxes (Vulpes vulpes) and golden jackals (Canis aureus) in six districts of Serbia. J. Zoo Wildl. Med. 2017;48:703–707. doi: 10.1638/2016-0169.1. [DOI] [PubMed] [Google Scholar]
- Drygala F., Stier N., Zoller H., Boegelsack K., Mix H.M., Roth M. Habitat use of the raccoon dog (Nyctereutes procyonoides) in North-Eastern Germany. Mamm. Biol. 2008;73:371–378. [Google Scholar]
- EFSA The European Union one health 2020 Zoonoses report. EFSA J. 2021;19:6971. doi: 10.2903/j.efsa.2021.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission Status of large carnivore populations in Europe 2012-2016. Nature and Biodiversity. 2019. https://ec.europa.eu/environment/nature/conservation/species/carnivores/conservation_status.htm
- European Environment Agency Landscape fragmentation in Europe. EEA Report No 2/2011. 2011. https://www.eea.europa.eu/publications/landscape-fragmentation-in-europe
- Famerée L., Cotteleer C., Van Den Abbeele O. Trichinosis in Belgium. Apropos of a familial epidemic following consumption of wild boar meat. Rev. Med. Liege. 1979;34:464–473. (in French) [PubMed] [Google Scholar]
- Frey C.F., Schuppers M.E., Müller N., Ryser-Degiorgis M.P., Gottstein B. Assessment of the prevalence of Trichinella spp. in red foxes and Eurasian lynxes from Switzerland. Vet. Parasitol. 2009;159:295–299. doi: 10.1016/j.vetpar.2008.10.060. [DOI] [PubMed] [Google Scholar]
- Frey C.F., Basso W.U., Zürcher-Giovannini S., Marti I., Borel S., Guthruf S., et al. The golden jackal (Canis aureus): a new host for Echinococcus multilocularis and Trichinella britovi in Switzerland. Schweiz. Arch. Tierheilkd. 2022;164:71–78. doi: 10.17236/sat00338. [DOI] [PubMed] [Google Scholar]
- Furnas B.J., Goldstein B.R., Figura P.J. Intermediate fire severity diversity promotes richness of forest carnivores in California. Diversity Distributions. 2021:1–13. [Google Scholar]
- Garkavi B.L. Species of Trichinella isolated from wild animals. Veterinariya. 1972;10:90–91. [PubMed] [Google Scholar]
- Gerbens-Leenes P.W., Mekonnen M.M., Hoekstra A.Y. The water footprint of poultry, pork and beef: a comparative study in different countries and production systems. Water Res Ind. 2013;1:25–36. [Google Scholar]
- Hansen C.P., Parsons A.W., Kays R., Millspaugh J.J. Does use of backyard resources explain the abundance of urban wildlife? Frontiers Ecol. Evolution. 2020;8:1–12. [Google Scholar]
- Huber K. Resilience Strategies for Wildfire. 2018. https://www.c2es.org/wp-content/uploads/2018/11/resilience-strategies-for-wildfire.pdf
- Jernelöv A. Springer; 2017. The Long-Term Fate of Invasive Species. Aliens Forever or Integrated Immigrants with Time? pp. 177–195. [Google Scholar]
- Jourdan P.M., Lamberton P.H.L., Fenwick A., Addiss D.G. Soil-transmitted helminth infections. Lancet. 2018;391:252–265. doi: 10.1016/S0140-6736(17)31930-X. [DOI] [PubMed] [Google Scholar]
- Kärssin A., Häkkinen L., Niin E., Peik K., Vilem A., Jokelainen P., et al. Trichinella spp. biomass has increased in raccoon dogs (Nyctereutes procyonoides) and red foxes (Vulpes vulpes) in Estonia. Parasit. Vectors. 2017;10:609. doi: 10.1186/s13071-017-2571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärssin A., Häkkinen L., Vilem A., Jokelainen P., Lassen B. Trichinella spp. in Wild Boars (Sus scrofa), Brown Bears (Ursus arctos), Eurasian Lynxes (Lynx lynx) and Badgers (Meles meles) in Estonia, 2007-2014. Animals (Basel) 2021;11:183. doi: 10.3390/ani11010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G., Wardell-Johnson G.W. Refugia: keys to climate change management. Glob. Change Biol. 2012;18:2389–2391. [Google Scholar]
- Kirjušina M., Deksne G., Marucci G., Bakasejevs E., Jahundoviča I., Daukšte A., et al. A 38-year study on Trichinella spp. in wild boar (Sus scrofa) of Latvia shows a stable incidence with an increased parasite biomass in the last decade. Parasit. Vectors. 2015;8:137. doi: 10.1186/s13071-015-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Olvera J.R., Vives L., Serrano E., Fernández-Sirera L., Picart L., Rossi L., et al. Trichinella sp. in red foxes (Vulpes vulpes) from Catalonia, NE Spain. Parasitol. Res. 2011;108:1589–1591. doi: 10.1007/s00436-011-2254-2. [DOI] [PubMed] [Google Scholar]
- Madsen H. In: Trichinellosis. Kim C.W., editor. Intext Educational Publishers; New York: 1974. The principles of the epidemiology of trichinellosis with a new view on the life cycle; pp. 615–638. [Google Scholar]
- Manlick P.J., Pauli J.N. Human disturbance increases trophic niche overlap in terrestrial carnivore communities. PNAS. 2020;117:26842–26848. doi: 10.1073/pnas.2012774117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazza V. La trichinosi della volpe in Italia. Arch. Vet. It. 1960;11:507–566. [Google Scholar]
- Marinculić A., Gaspar A., Duraković E., Pozio E., La Rosa G. Epidemiology of swine trichinellosis in the Republic of Croatia. Parasite. 2001;8(2S):S92–S94. doi: 10.1051/parasite/200108s2092. [DOI] [PubMed] [Google Scholar]
- Mayer-Scholl A., Reckinger S., Schulze C., Nöckler K. Study on the occurrence of Trichinella spp. in raccoon dogs in Brandenburg, Germany. Vet. Parasitol. 2016;231:102–105. doi: 10.1016/j.vetpar.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Messiaen P., Forier A., Vanderschueren S., Theunissen C., Nijs J., Van Esbroeck M., et al. Outbreak of trichinellosis related to eating imported wild boar meat, Belgium, 2014. Euro Surveill. 2016;21:30341. doi: 10.2807/1560-7917.ES.2016.21.37.30341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell K.D., Pozio E. Worldwide occurrence and impact of human trichinellosis, 1986-2009. Emerg. Infec. Dis. 2011;17:2194–2202. doi: 10.3201/eid1712.110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen A., Interisano M., Isomursu M., Heikkinen P., Tonanzi D., Oivanen L., et al. Trichinella spiralis prevalence among wildlife of a boreal region rapidly reduced in the absence of spillover from the domestic cycle. Vet. Parasitol. 2018;262:1–5. doi: 10.1016/j.vetpar.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Owen I.L., Awui C., Langelet E., Soctine W., Reid S. The probable role of cannibalism in spreading Trichinella papuae infection in a crocodile farm in Papua New Guinea. Vet. Parasitol. 2014;203:335–338. doi: 10.1016/j.vetpar.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Pauli J.N., Zuckerberg B., Whiteman J.P., Porter W. The subnivium: a deteriorating seasonal refugium. Front. Ecol. Environ. 2013;11:260–267. [Google Scholar]
- Poore J., Nemecek T. Reducing food's environmental impacts through producers and consumers. Science. 2018;360:987–992. doi: 10.1126/science.aaq0216. [DOI] [PubMed] [Google Scholar]
- Pozio E. Searching for Trichinella: not all pigs are created equal. Trends Parasitol. 2014;30:4–11. doi: 10.1016/j.pt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Pozio E. Trichinella spp. imported with live animals and meat. Vet. Parasitol. 2015;213:46–55. doi: 10.1016/j.vetpar.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Pozio E. Trichinella pseudospiralis an elusive nematode. Vet. Parasitol. 2016;231:97–101. doi: 10.1016/j.vetpar.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Pozio E. Adaptation of Trichinella spp. for survival in cold climates. Food Waterborne Parasitol. 2016;4:4–12. [Google Scholar]
- Pozio E. In: Foodborne Parasites. second edition. Ortega Y.R., Sterling C.R., editors. Springer International Publishing; 2018. Trichinella and other foodborne nematodes; pp. 175–215. [Google Scholar]
- Pozio E. How globalization and climate change could affect foodborne parasites. Exp. Parasitol. 2020;208 doi: 10.1016/j.exppara.2019.107807. [DOI] [PubMed] [Google Scholar]
- Pozio E. In: Trichinella and trichinellosis. Bruschi F., editor. Academic Press; London, San Diego, Cambridge: 2021. Epidemiology; pp. 185–265. [Google Scholar]
- Pozio E., Zarlenga D.S. New pieces of the Trichinella puzzle. Int. J. Parasitol. 2013;43:983–997. doi: 10.1016/j.ijpara.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Pozio E., Owen I.L., Marucci G., La Rosa G. Inappropriate feeding practice favors the transmission of Trichinella papuae from wild pigs to saltwater crocodiles in Papua New Guinea. Vet. Parasitol. 2005;127:245–251. doi: 10.1016/j.vetpar.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Pozio E., Celli M., Ludovisi A., Interisano M., Amati M., Gómez-Morales M.A. Animal welfare and zoonosis risk: anti-Trichinella antibodies in breeding pigs farmed under controlled housing conditions. Parasit. Vectors. 2021;14:417. doi: 10.1186/s13071-021-04920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribicich M., Gamble H.R., Rosa A., Bolpe J., Franco A. Trichinellosis in Argentina: an historical review. Vet. Parasitol. 2005;132:137–142. doi: 10.1016/j.vetpar.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Rossi L., Interisano M., Deksne G., Pozio E. The subnivium, a haven for Trichinella larvae in host carcasses. Int. J. Parasitol. Parasites Wildl. 2019;8:229–233. doi: 10.1016/j.ijppaw.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A., Gamble H.R., Dupouy-Camet J., Khazan H., Bruschi F. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol. 2017;64:65–71. doi: 10.1016/j.fm.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Sandoval C.M., Gutiérrez R., Luna S., Amaya M., Esteban L., Ariza H., et al. High density of Rhodnius prolixus in a rural house in Colombia. Trans. Roy. Soc. Trop. Med. Hyg. 2000;94:372–373. doi: 10.1016/s0035-9203(00)90107-x. [DOI] [PubMed] [Google Scholar]
- Smil V. Harvesting the biosphere: the human impact. Popul. Dev. Rev. 2011;37:613–636. doi: 10.1111/j.1728-4457.2011.00450.x. [DOI] [PubMed] [Google Scholar]
- Széll Z., Marucci G., Pozio E., Sréter T. Echinococcus multilocularis and Trichinella spiralis in golden jackals (Canis aureus) of Hungary. Vet. Parasitol. 2013;197:393–396. doi: 10.1016/j.vetpar.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Takahashi Y. In: Trichinella and trichinellosis. Bruschi F., editor. Academic Press; London, Sand Diego, Cambridge: 2021. Biology of Trichinella; pp. 77–101. [Google Scholar]
- Theeuwes N.E., Steeneveld G.J., Ronda R.J., Holtslag A.A.M. A diagnostic equation for the daily maximum urban heat island effect for cities in northwestern Europe. Int. J. Climatol. 2017;37:443–454. [Google Scholar]
- Weka R.P., Kamani J., Cogan T., Eisler M., Morgan E.R. Overview of Taenia solium cysticercosis in West Africa. Acta Trop. 2019;190:329–338. doi: 10.1016/j.actatropica.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Worley D.E., Seesee F.M., Espinosa R.H., Sterner M.C. Survival of sylvatic Trichinella spiralis isolates in frozen tissue and processed meat products. J. Am. Vet. Med. Assoc. 1986;189:1047–1049. [PubMed] [Google Scholar]
- Worley D.E., Zarlenga D.S., Seesee F.M. Freezing resistance of a Trichinella spiralis nativa isolate from a gray wolf, Canis lupus, in Montana (USA) with observations on genetic and biological characteristics of the biotype. J. Helminthol. Soc. Washington. 1990;57:57–60. [Google Scholar]
- Zarlenga D., Thompson P., Pozio E. Trichinella species and genotypes. Res. Vet. Sci. 2020;133:289–296. doi: 10.1016/j.rvsc.2020.08.012. [DOI] [PubMed] [Google Scholar]