Figure 1.
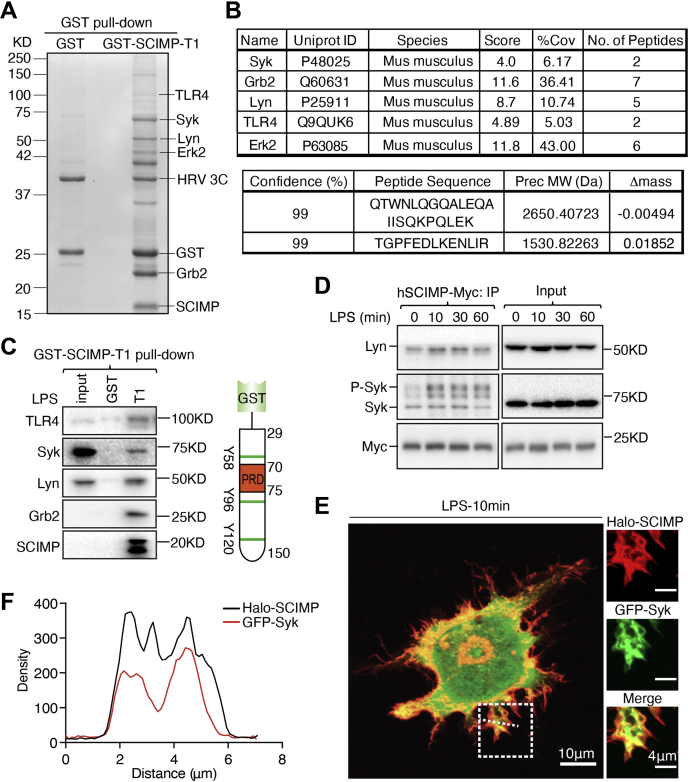
Syk is a novel binding partner of SCIMP.A, pull-downs from LPS-activated RAW264.7 cell extracts. GST-SCIMP-T1 was immobilized to GSH-Sepharose to capture its binding partners in macrophage lysates. Bound proteins were eluted by a protease cleavage elution (PCE) method (28) and separated by SDS–PAGE. Excised bands were identified by LC/MS/MS. B, list of the top hits from the LC/MS/MS analysis of GST-SCIMP-T1 pull-downs. C, GST-SCIMP-T1 pull-down from cell extracts of LPS activated, SCIMP-deficient RAW264.7 cells stably reconstituted with WT-V5-SCIMP. Syk and other binding partners are detected in the pull-down by immunoblotting. D, immunoprecipitation of myc-SCIMP-WT expressed in human PMA-differentiated THP-1 cells (56). Cells were treated with LPS over a time course and lysates made at each time point were used for IP with a Myc antibody and Syk was detected by immunoblotting. Immunoblotting of Lyn was used as a control. E and F, RAW264.7 cells cotransfected with Halo-SCIMP (red) and GFP-Syk (green). Cells were treated with Halo-549 ligand and LPS ligand for 15 min and 10 min, respectively, prior to imaging. The scale bar represents 10 μm or 4 μm. Intensity profiles of surface ruffles across the membrane (dash white line) is quantified for individual channels. Panels C–E are representative of three independent experiments.IP, immunoprecipitation; LPS, lipopolysaccharides; PMA, phorbol 12-myristate 13-acetate; Syk, Spleen tyrosine kinase.