Figure 19.
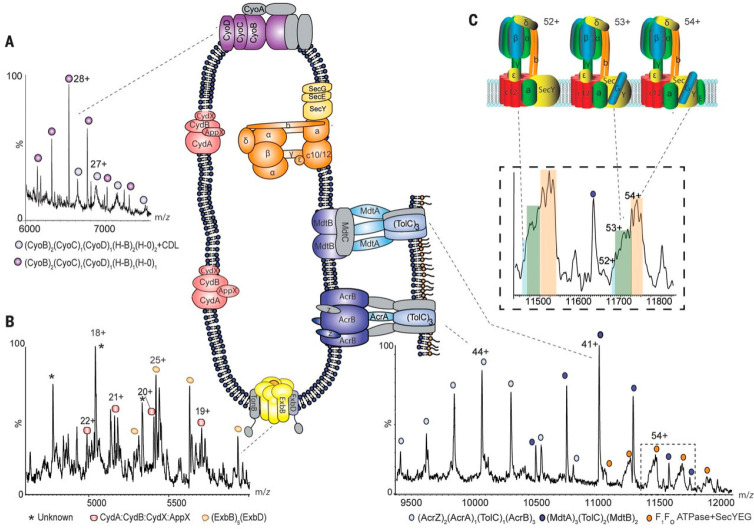
Native MS of membrane-embedded protein complexes analyzed directly from their native environment. Regions of the mass spectrum recorded for inner membranes from E. coli yield cytochromes, the Ton complex multidrug transporters, and the intact ATP synthase in complex with the SecYEG translocon. (A, B) Expanded regions of the spectrum assigned to cytochrome bo3 and cytochrome bd oxidase, showing peak splitting due to binding of quinol and heme groups. The pentameric ExbB complex (with one copy of ExbD in the center of the pore) that forms part of the TonB complex is also observed (yellow). (C) High-m/z region of the mass spectrum assigned to the multidrug efflux pumps AcrAB and MdtAB and the intact ATP synthase. Expansion of peaks assigned to the ATPase reveals binding of the SecY (blue), SecYG (green), and SecYEG (orange) charge states 52+, 53+, and 54+. Complexes observed in mass spectra are shown schematically, with subunits that have dissociated shown in gray. Reproduced with permission from ref (323). Copyright 2018 Chorev et al.