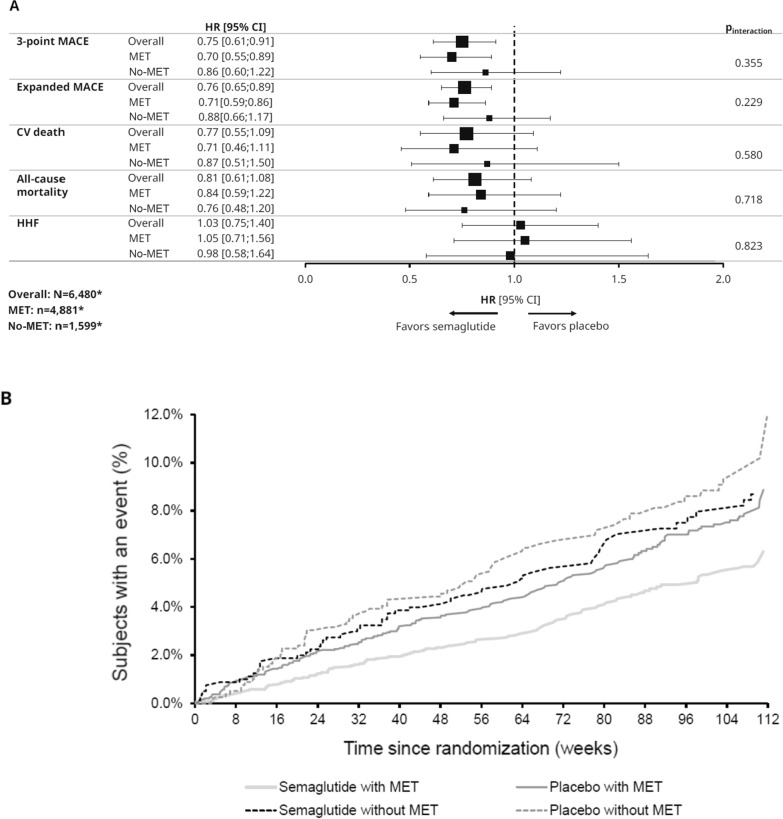
Fig. 2.
A CV outcomes/mortality by baseline metformin use (adjusted analysis); B Kaplan–Meier curves of primary outcome by baseline metformin. Figures (A) and (B) compare semaglutide with placebo. *n-numbers are based on the FAS; the number of subjects included for each endpoint analysis differed according to data availability. A Analyses for SUSTAIN 6 and PIONEER 6 are based on a Cox proportional hazards model with treatment (semaglutide, placebo) by metformin subgroup as fixed factors, stratified by trial and CV risk group (established CVD and/or CKD vs risk factors), and adjusted by baseline variables: sex (male vs female), smoker (current smoker, previous smoker, never smoked), previous MI/stroke/TIA (yes vs no), region (European Union, North America, other), antidiabetic treatment (yes vs no), diabetes duration, eGFR-MDRD and age. CI confidence interval, CKD chronic kidney disease, CV cardiovascular, CVD cardiovascular disease, eGFR-MDRD estimated glomerular filtration rate-modification of diet in renal disease, FAS full analysis set, HbA1c glycated hemoglobin, HHF hospitalization for heart failure, HR hazard ratio (semaglutide vs placebo), MACE major adverse cardiovascular event, MET metformin, MI myocardial infarction, TIA transient ischemic attack