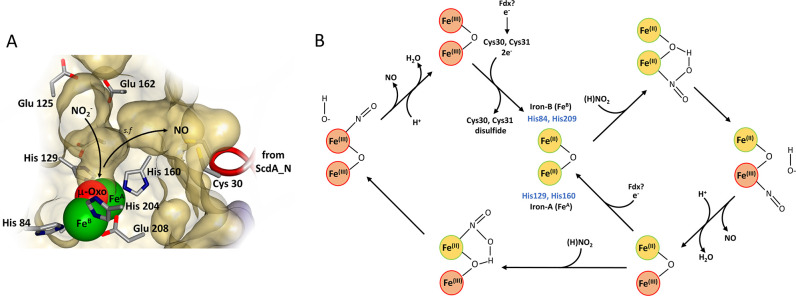
Figure 7.
Mechanism of YtfE-catalyzed nitrite reduction. (A) The hydrophilic and hydrophobic channels connect to the di-iron cavity to create a Y-shaped cavity (1.4 Å probe radius). The surface-exposed hydrophilic channel, ringed by Glu125, Glu159, and Glu162, provides substrate access to iron A (FeA) and the μ-oxo-bridge of the di-iron center. Post catalysis, the resulting NO departs via the hydrophobic channel and selection filter (s.f.) toward the thiolates of the ScdA_N domain. We note that the movement of the ScdA_N domain controls the access of exogenous NO to the di-iron site. Molecular graphics were analyzed and created using Biovia Discovery Studio (Dassault Systèmes). (B) Proposed mechanism of catalysis. Nitrite (or HNO2) binds to FeA of di-ferrous YtfE via nitrogen, promoting the oxidation of FeA and fission of HNO2 into NO and OH–. Departure of NO and OH– (as H2O following protonation) from the mixed-valent site is followed by reduction back to the di-ferrous state or further reaction with a second (H)NO2 at the remaining Fe2+ ion. This results in a di-ferric form, which can be reduced back to the di-ferrous form by electrons from Cys30/31, resulting in a disulfide bond. Catalysis requires a supply of electrons, possibly from a ferredoxin (Fdx), which could feed into the di-iron site directly or indirectly via Cys30/31.