Figure 9.
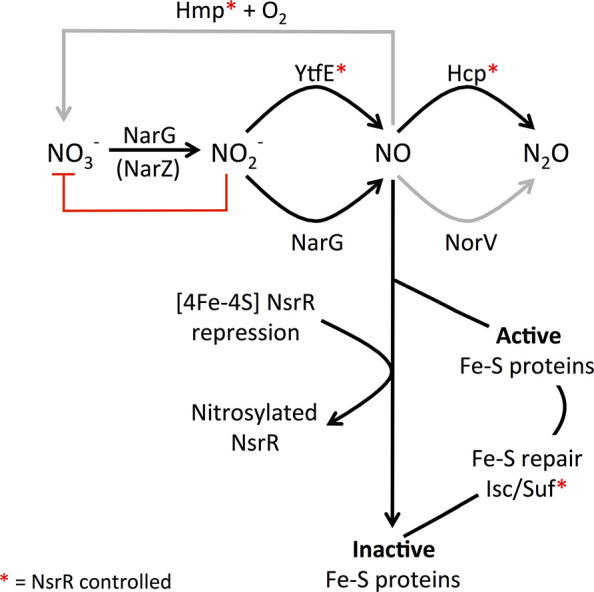
Proposed role of YtfE in the management of endogenous nitrosative stress in E. coli. Nitrate reductases (NarG and, probably, NarZ) increase the cytoplasmic concentration of nitrite. Accumulated nitrite competes with nitrate at the active site of NarG and therefore impairs nitrate reduction and growth (red line). We propose that YtfE reduces nitrite to NO to optimize nitrate respiratory growth. The NO generated by YtfE can inactivate Fe-S proteins, including the global transcriptional repressor [4Fe-4S] NsrR. Nitrosylated NsrR is not a competent repressor, leading to the derepression of genes encoding the response to nitrosative stress (indicated by red asterisks), including the high-affinity NO reductase Hcp and its cognate reductase Hcr. Thus, YtfE and NsrR can be thought of as a two-component system that monitors cytosolic nitrite concentrations, resulting in responses, including the induction of hcp-hcr, that promote efficient nitrate respiratory growth, with Hcp acting predominantly under anaerobic conditions when the concentration of NO is <1 μM. The alternative NO reductases, Hmp and NorV, lower the cytosolic concentration of NO under different regimes, microaerobic (Hmp) or anaerobic with >1 μM NO (NorV), respectively, (gray arrows). Under some conditions, NirB is deployed to convert nitrite to ammonium (see the main text). Inactivated Fe-S proteins are repaired by the Isc and/or Suf systems.
