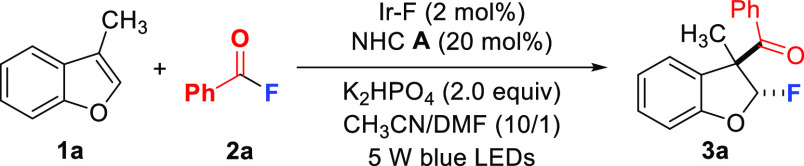
Table 1. Reaction Optimizationa.
| entry | variation from the standard condition | yield of 3a (%)b |
|---|---|---|
| 1 | none | 73 (70)c |
| 2 | Ru(bpy)3(PF6)2 instead of Ir–F | ND |
| 3 | 9-Mesityl-10-methylacridinium instead of Ir–F | ND |
| 4 | CH3CN instead of CH3CN/DMF | 50 |
| 5 | CH3CN/Acetone instead of CH3CN/DMF | 61 |
| 6 | Cs2CO3 instead of K2HPO4 | 40 |
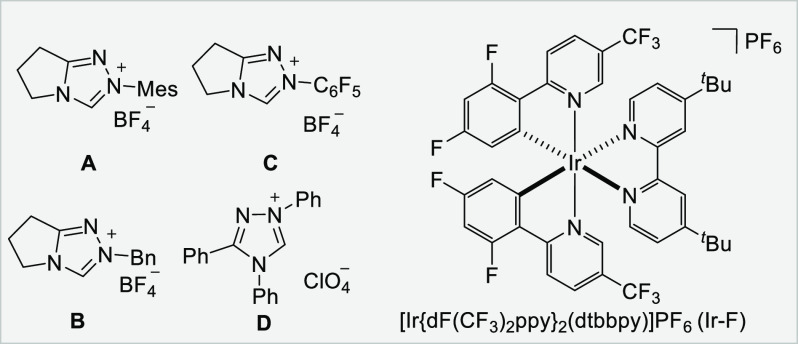
| 7 | NHC B instead of NHC A | 2 |
| 8 | NHC C instead of NHC A | 35 |
| 9 | NHC D instead of NHC A | ND |
| 10 | no light irradiation | ND |
| 11 | no NHC catalyst | ND |
| 12 | no photocatalyst | ND |
Reaction conditions: 1a (0.1 mmol), 2a (0.4 mmol), NHC A (20 mol %), Ir–F (2 mol %), K2HPO4 (2.0 equiv), and CH3CN/DMF (1 mL/0.1 mL) under irradiation with 5 W blue LEDs for 24 h, 15:1 d.r.
Yields were determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.