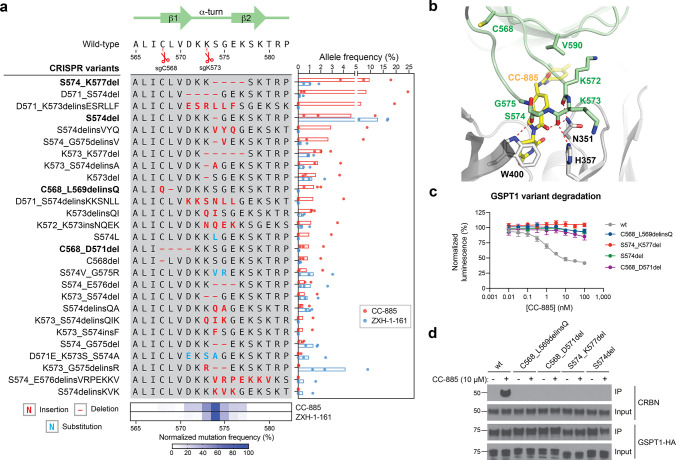
Figure 2.
CC-885 resistance mutations alter the GSPT1 β-hairpin structural degron and impair GSPT1 degradation. (a) Left: Schematic shows the coding variants of the most abundant in-frame mutations enriched in the β-hairpin structural degron of GSPT1 (>1% frequency in any condition). Right: Bar plot showing frequency (%, x axis) of each variant. Bars represent the mean across three replicate treatments, and dots show the individual replicate values. Bottom: Heat map showing normalized mutational frequency (y axis, %) by sequence position (x axis). Mutational frequency was normalized as a percentage of the total frequency of the displayed variants. (b) Structural view of the CC-885-CRBN-GSPT1 ternary complex, with key residues in CRBN (gray) and GSPT1 (green) highlighted. Carbon atoms of CC-885 are depicted in yellow (PDB: 5HXB). (c) Dose–response curves for wt and mutant HiBiT-GSPT1-HA cellular protein levels, as indicated by vehicle-normalized luminescence (y axis, %), in HEK293T cells treated with CC-885 for 6 h. Data represent mean ± s.e.m. across three technical replicates. One of two independent experiments is shown. (d) Immunoblots showing co-IP of GSPT1-HA wt and mutant variants with CRBN after vehicle or CC-885 treatment (10 μM, 2 h) in transiently transfected HEK293T cells. All cells were first pretreated with MLN-4924 (1 μM, 3 h) prior to vehicle or CC-885 treatment. Co-IP was performed using anti-HA antibody. One of two independent replicates is shown.