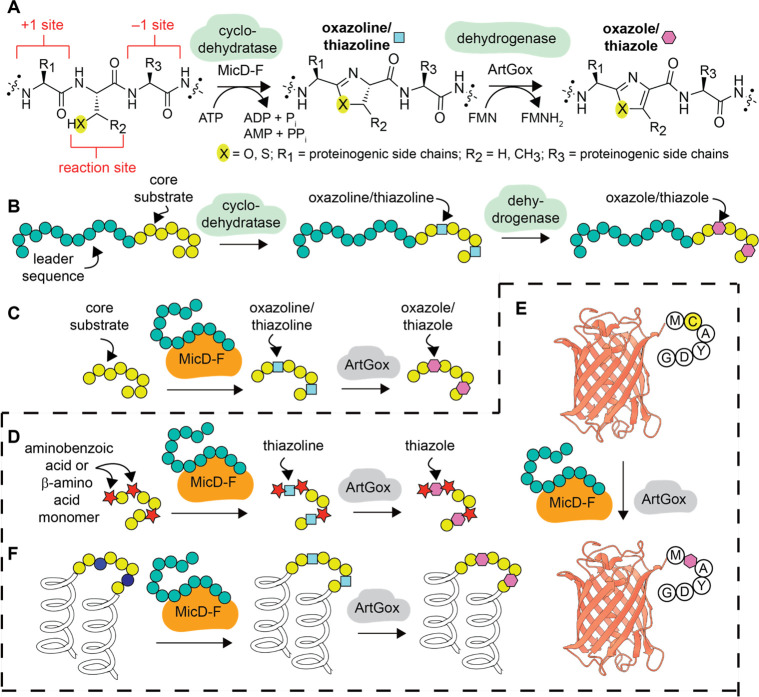
Figure 1.
Overview of cyclodehydratase/dehydrogenase chemistry. (A) Scheme illustrating the natural conversion of a serine, threonine, or cysteine-containing polypeptide into a oxazoline or thiazoline-containing product through the action of MicD21 and subsequent dehydrogenation into an oxazole or thiazole through the action of ArtGox.24 (B) Natural substrates for MicD34 and ArtGox35 consist of a core sequence that includes the reaction site and an extended upstream leader sequence. (C) Fusion of the leader sequence to the N-terminus of MicD generates a constitutively activated enzyme MicD-F that processes leaderless substrates.21 (D) This work: MicD-F and ArtGox accept leaderless polypeptide substrates containing diverse non-α-amino acid monomers, including aminobenzoic acid derivatives, at the +1 and −1 sites. MicD-F and ArtGox can also install thiazoline and thiazole linkages within leaderless globular proteins such as (E) mCherry and (F) Rop.