Figure 6.
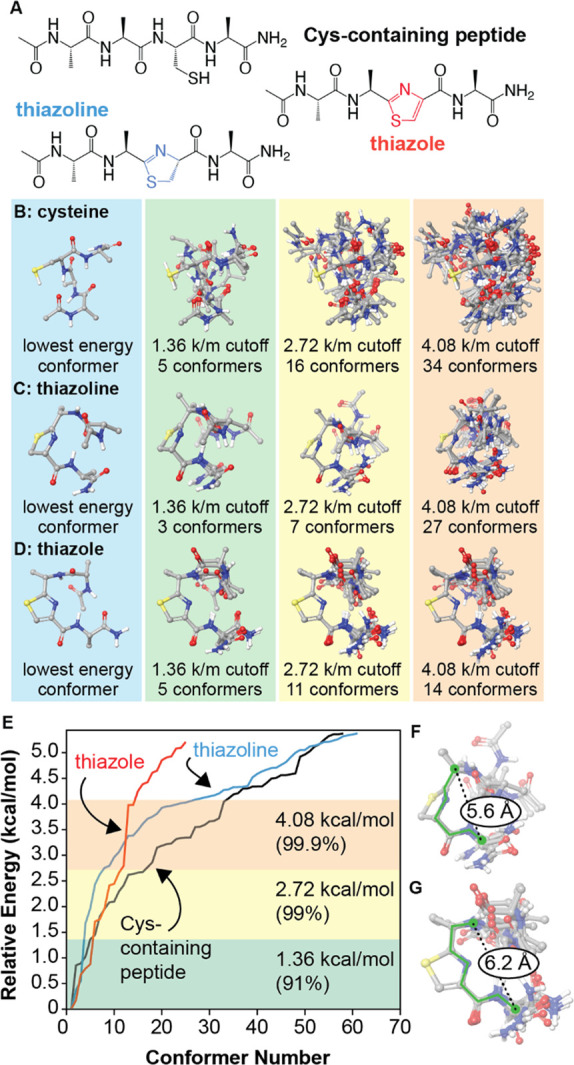
Conformational effects of thiazoline and thiazole formation. (A) The open chain and cyclized analogues Ac-AACA-NH2 were examined. Initial conformational searches were conducted using MacroModel (OPLS4 force field). All species within 4 kcal/mol of the global minimum were geometry optimized using DFT (B3LYP/6-31G**, SM8 solvent model) and reranked. (B–E) Lowest energy conformers are superimposed for different energy cutoff values. (F) A rigid six-bond motif (green) describes all identified conformers within 2.72 kcal/mol of the global minimum for the thiazoline. (G) A similar seven-bond motif describes the thiazole conformers. k/m = kcal/mol. For images of all structures within 4.08 kcal/mol of each global minimum, see Supporting Information.
