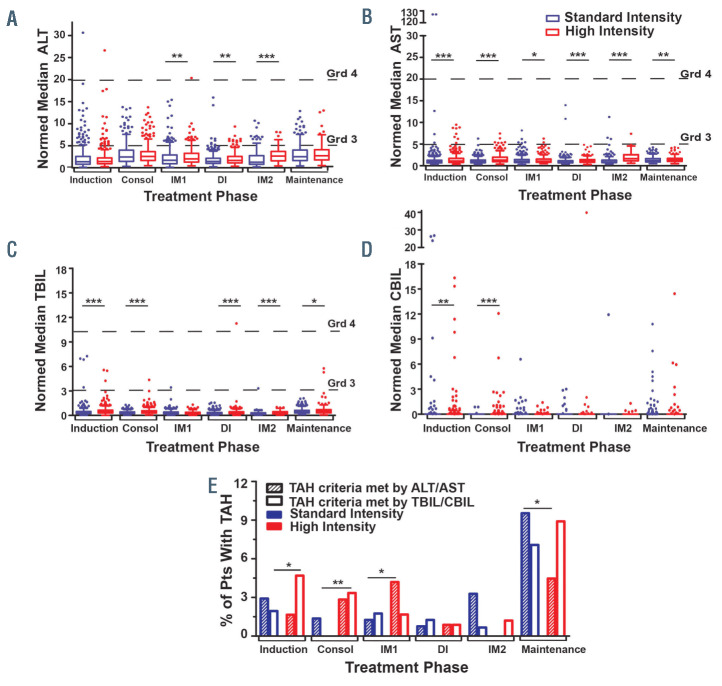
Figure 1.
Trends in hepatic laboratory values, including treatment-associated hepatotoxicity, during acute lymphoblastic leukemia therapy by treatment intensity. (A-D) The normed median hepatic laboratory value (HL) of subjects by each HL are represented by box and whisker plots, with outliers shown in the dots, subjects given standard intensity treatment in blue, and those given high intensity treatment in red. (A) Normed median alanine aminotransaminase (ALT, SGPT). (B) Normed median aspartate aminotransaminase (AST, SGOT). (C) Normed median total bilirubin (TBIL). (D) Normed median conjugated bilirubin (CBIL). Dashed lines indicate thresholds of CTCAE v5.0 grading for grade 3 or grade 4 ALT, AST, or TBIL as follows: ALT/AST: Grd 3= 5-20x upper limit of normal (ULN), Grd 4= >20x ULN. TBIL: Grd 3= 3-10x ULN, Grd 4= >10x ULN. (E) Percentage of patients with treatment-associated hepatotoxicity (TAH) by treatment intensity over all courses of therapy. *P<0.05, **P=0.001-<0.01, ***P<0.001, comparing standard vs. high intensity groups. For (E), comparisons were made between TAHALT/ AST of each intensity group (hashed bars) and between TAH-TBIL/CBIL of each intensity group (open bars) for each treatment phase. Consol: consolidation; IM1: interim maintenance 1; DI: delayed intensification; IM2i: interim maintenance 2.