Figure 1.
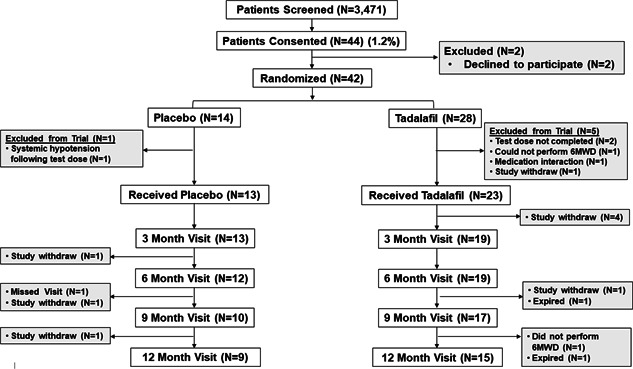
Enrollment, randomization, and follow up. Veterans with chronic obstructive pulmonary disease and pulmonary hypertension diagnosed by right heart catheterization were considered for enrollment in this study randomizing patients to daily treatment with placebo or the phosphodiesterase type 5 inhibitor therapy tadalafil (40 mg daily). The primary endpoint was changed in 6‐min walk distance (6‐MWD) from baseline at 12 months. Secondary endpoints included change in mean pulmonary artery pressure, pulmonary vascular resistance, 6‐MWD, and dyspnea burden from baseline at 6 months
