Abstract
Balloon pulmonary angioplasty (BPA) for chronic thromboembolic pulmonary hypertension (CTEPH) or pulmonary disease (CTEPD) is performed worldwide. High mean pulmonary arterial pressure (mPAP) during BPA is associated with aggravation of procedure‐related complications. Inhaled nitric oxide (NO) acts as a pulmonary vasodilator in CTEPH patients. The aim of this retrospective observational study was to evaluate the effects of inhaled NO in CTEPH/CTEPD patients during BPA. We assessed hemodynamic changes and the frequency of procedure‐related complications with or without NO inhalation. We performed 338 consecutive BPA in 72 CTEPH/CTEPD patients between April 2016 and November 2020. Since December 2019, all 16 patients (72‐BPA sessions) inhaled NO during the procedure as a routine. Inhaled NO significantly reduced mPAP from 31.3 ± 8.5 to 27.2 ± 6.8 mmHg (p < 0.001) without lowering systemic blood pressure (systolic: 139.9 ± 19.9 vs. 135.3 ± 21.8 mmHg; p = 0.247, diastolic: 82.8 ± 13.0 vs. 79.0 ± 9.3 mmHg; p = 0.085, before vs. after NO inhalation, respectively). Procedure‐related complications were observed in 46 sessions (13.6%). The incidence of hemoptysis was significantly higher in the NO group than in the non‐NO group (20.8% vs. 10.9%; p = 0.031). In contrast, all fatal complications (death and use of positive pressure ventilation) occurred in the non‐NO group, but the difference was not statistically significant (0 sessions [0.0%] vs. 7 sessions [2.6%], p = 0.353). Inhaled NO acts as a selective pulmonary vasodilator in CTEPH/CTEPD patients during the BPA procedure.
Keywords: balloon pulmonary angioplasty, chronic thromboembolic pulmonary hypertension, nitric oxide
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is caused by organized thrombi in pulmonary arteries and is classified as Group 4 pulmonary hypertension. 1 , 2 Balloon pulmonary angioplasty (BPA) has emerged as a promising alternative to pulmonary endarterectomy for inoperable CTEPH patients. 3 , 4 Recently, BPA has been performed to improve not only prognosis but also the quality of life (QOL). 3 , 4 , 5 , 6 , 7 Patients with symptomatic chronic thromboembolic pulmonary disease without pulmonary hypertension at rest (CTEPD) increasingly undergo BPA to improve their symptoms. 3 , 8 Currently, for complete revascularization, distal lesions (in subsegmental or more distal pulmonary arteries) can be targets for BPA. 3 , 4 Procedure‐related pulmonary bleeding is the main cause of complications of BPA, and these aggressive BPA procedures sometimes promote procedure‐related complications. 9 , 10 To prevent life‐threatening complications, such as death and severe respiratory failure requiring positive pressure ventilation, it is important to establish safer procedures. The mean pulmonary arterial pressure (mPAP) during BPA has been reported to be associated with aggravation of procedure‐related complications. 11 Therefore, lowering pulmonary arterial pressure during the BPA procedure may be an effective strategy to reduce the severity of complications. Nitric oxide (NO) inhalation acts as a selective pulmonary vasodilator and temporarily lowers pulmonary arterial pressure. Inhaled NO also acts as a pulmonary vasodilator even in patients with CTEPH caused by organized thrombi. 13 , 14
This study was a hypothesis‐generating pilot study to assess the effects of NO inhalation on hemodynamic changes and the incidence of procedure‐related complications during BPA in patients with CTEPH/CTEPD.
METHODS
Patient selection
This retrospective observational study was conducted based on the medical records at our institution. The study population consisted of consecutive CTEPH/CTEPD patients who underwent BPA at our institute between April 2016 and November 2020.
In this study, the diagnosis of CTEPH/CTEPD was based on organized thrombi in pulmonary arteries by contrast‐enhanced lung computed tomography (CT), perfusion defects by perfusion lung scintigraphy, and pulmonary hypertension with right heart strain by right heart catheterization (RHC) and echocardiography. Other causes of pulmonary hypertension were ruled out by systemic CT, chest X‐ray, pulmonary function tests, blood tests, echocardiography, and RHC. RHC was performed using Swan–Ganz catheters. Right atrial pressure, right ventricular pressure, pulmonary arterial pressure, mPAP, pulmonary capillary wedge pressure (PCWP), cardiac output and index (CI), and pulmonary vascular resistance (PVR) were measured. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of our institute (H20001). The comprehensive agreement was obtained from all patients and opt‐out from the website of our institute provided the target patients with the opportunity to refuse participation in the present study.
NO inhalation
Since December 2019, all consecutive BPA procedures for CTEPH/CTEPD patients at our institute involved NO inhalation during the procedure as a routine (in the NO era). Even if it was not the first session for the patients, NO inhalation was performed, and the data at the first session in the NO era were used for hemodynamic analysis (Figure 1). NO was supplied from an NO inhaler (INOflo SD®; Mallinckrodt Pharma K.K.) to patients to maintain 20 ppm with 4 L/min oxygen. RHC using Swan–Ganz catheters was performed before NO inhalation and 10 min after the start of inhalation. The BPA procedure started after the measurement was completed.
Figure 1.
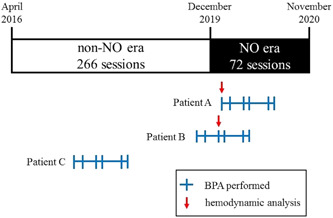
Study protocol. We performed 338 consecutive BPA procedures in 72 CTEPH/CTEPD patients during the study period. Fifty‐six patients (266 sessions) undergoing BPA before December 2019 did not inhale NO during the procedure (the non‐NO era), and 16 patients (72 sessions) were treated in the NO era. We analyzed hemodynamic changes by NO inhalation as follows. Patient A: all BPA sessions were performed in the NO era. We analyzed hemodynamic changes by NO inhalation from the first session's hemodynamic data. Patient B: BPA sessions were performed in both the non‐NO and NO eras. We analyzed hemodynamic changes by NO inhalation from the third (first time in the NO era) session's hemodynamic data. Patient C: all BPA sessions were performed in the non‐NO era. Accordingly, we did not analyze hemodynamic changes by NO inhalation. BPA, balloon pulmonary angioplasty; CTEPD, chronic thromboembolic pulmonary disease; CTEPH, chronic thromboembolic pulmonary hypertension; NO, nitric oxide
BPA procedure
An 8.2 or 9Fr sheath was inserted into the right internal jugular vein or right common femoral vein. From the sheath, a 6Fr 70 cm long guiding sheath (ParentPlus 60; Medikit) was inserted into the right or left main pulmonary artery. All BPA procedures were performed using a 6Fr catheter inserted through a 6Fr long guiding sheath. After advancing the catheter into the target pulmonary arteries, contrast media was injected to visualize lesions. The 0.014‐inch guidewire was advanced to the distal part of the target vessel crossing the lesions, and appropriately sized balloon catheters were used to dilate the lesions. During the BPA procedure, unfractionated heparin was administered to maintain a target activated clotting time of 180–250 s during each procedure.
Assessment of complications
All adverse events of BPA that occurred during the procedure or up to 48 h later were recorded. From these events, procedure‐related complications (death, use of positive pressure ventilation, and hemoptysis) were extracted and analyzed.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation. Categorical variables are presented as totals or proportions (percentages). The two‐sample paired or unpaired Student's t test or Mann–Whitney U test was used to compare continuous variables. The χ 2 or Fisher exact test was used to compare categorical variables. Two‐sided p < 0.05 was considered statistically significant. SPSS Statistics ver. 23 (IBM Japan) was used for analysis.
RESULTS
Subjects
During the study period, we performed 338 consecutive BPA procedures in 72 CTEPH/CTEPD patients. Fifty‐six patients (266 sessions) undergoing BPA before December 2019 did not inhale NO during the procedure (the non‐NO group), and 16 patients (72 sessions) were included in the NO era (the NO group) (Figure 1). The patients' baseline characteristics are shown in Table 1. There were no significant differences in hemodynamic status at baseline between the NO group and the non‐NO group. In particular, the mean pre‐BPA mPAP was 35.7 ± 9.3 mmHg in the non‐NO group and 34.3 ± 9.0 mmHg in the NO group (p = 0.597). A statistically similar percentage of the patients were taking oral pulmonary vasodilators between the non‐NO group and NO group (endothelin receptor antagonist; 12.5% vs. 6.25%; p = 0.674, phosphodiesterase‐5 inhibitor; 3.5% vs. 6.25%; p = 0.130, soluble guanylate cyclase stimulant; 35.7% vs. 18.8%; p = 0.239, prostacyclin analogue; 16.1% vs. 12.5%; p = 1.000, respectively).
Table 1.
Patients characteristics
| Total (n = 72) | Non‐NO group (n = 56) | NO group (n = 16) | p value | |
|---|---|---|---|---|
| Age (years) | 69.7 ± 12.9 | 70.9 ± 11.2 | 65.6 ± 17.3 | 0.262 |
| Male/female | 23/49 | 18/38 | 5/11 | 1.000 |
| History of CTEPH/CTED (years) | 3.0 ± 3.7 | 2.9 ± 3.2 | 3.3 ± 5.2 | 0.721 |
| Oral ERA | 8 | 7 (12.5%) | 1 (6.25%) | 0.674 |
| Oral PDE5i | 3 | 2 (3.5%) | 1 (6.25%) | 0.130 |
| Oral sGCs | 23 | 20 (35.7%) | 3 (18.8%) | 0.239 |
| Oral prostacyclin analog | 11 | 9 (16.1%) | 2 (12.5%) | 1.000 |
| Pre‐BPA PA s (mmHg) | 58.4 ± 18.5 | 58.8 ± 18.6 | 57.1 ± 18.6 | 0.749 |
| Pre‐BPA PA d (mmHg) | 20.1 ± 5.5 | 19.6 ± 5.6 | 22.0 ± 4.9 | 0.124 |
| Pre‐BPA mPAP (mmHg) | 34.6 ± 9.0 | 34.3 ± 9.0 | 35.7 ± 9.3 | 0.597 |
| Pre‐BPA PCWP m (mmHg) | 9.8 ± 3.8 | 9.6 ± 3.9 | 10.5 ± 3.1 | 0.396 |
| Pre‐BPA CI (L/min/m2) | 2.8 ± 0.7 | 2.7 ± 0.7 | 3.1 ± 0.8 | 0.069 |
| Pre‐BPA PVR (dyne/sec/cm5) | 475.4 ± 256.6 | 489.8 ± 235.7 | 426.1 ± 322.4 | 0.386 |
Abbreviations: BPA, balloon pulmonary angioplasty; CI, cardiac index; CTED, chronic thromboembolic disease; CTEPH, chronic thromboembolic pulmonary hypertension; ERA, endothelin receptor antagonist; mPAP, mean pulmonary arterial pressure; NO, nitric oxide; PA d, diastolic pulmonary arterial pressure; PA s, systolic pulmonary arterial pressure; PCWP m, mean pulmonary capillary wedge pressure; PDE5i, phosphodiesterase‐5 inhibitor; PVR, pulmonary vascular resistance; sGCs, soluble guanylate cyclase stimulant.
Hemodynamic changes due to NO inhalation
Sixteen patients (72 sessions) inhaled NO during BPA and were evaluated for hemodynamic status by RHC before and after inhalation. There were no adverse events due to NO inhalation in this study.
Hemodynamic changes by NO inhalation are shown in Table 2 and Figure 2. NO inhalation significantly reduced pulmonary arterial pressure (systolic; from 51.2 ± 16.6 to 43.3 ± 13.2 mmHg; p < 0.001, diastolic; from 19.2 ± 5.1 to 17.1 ± 3.1 mmHg; p = 0.012, mean; from 31.3 ± 8.5 to 27.2 ± 6.8 mmHg; p < 0.001, before to after NO inhalation, respectively). Alternatively, there were no significant differences in systemic blood pressure before and after NO inhalation (systolic; 139.9 ± 19.9 vs. 135.3 ± 21.8 mmHg; p = 0.247, diastolic; 82.8 ± 13.0 vs. 79.0 ± 9.3 mmHg; p = 0.085, before vs. after NO inhalation, respectively) (Figure 2). Cardiac index (from 2.9 ± 0.6 to 2.6 ± 0.9 L/min/m2; p = 0.001) and heart rate (from 75.3 ± 21.9 to 68.9 ± 14.7 bpm; p = 0.043) were decreased by NO inhalation.
Table 2.
Hemodynamic parameters pre and post nitric oxide
| Pre‐NO inhalation | Post‐NO inhalation | p value | |
|---|---|---|---|
| PAP s (mmHg) | 50.1 ± 16.6 | 43.6 ± 13.2 | <0.001 |
| PAP d (mmHg) | 19.2 ± 5.1 | 17.1 ± 3.7 | 0.012 |
| mPAP (mmHg) | 31.3 ± 8.5 | 27.2 ± 6.8 | <0.001 |
| PCWP m (mmHg) | 13.4 ± 4.8 | 12.2 ± 5.2 | 0.144 |
| CI (L/min/m2) | 2.9 ± 0.6 | 2.6 ± 0.7 | 0.001 |
| PVR (dyne/sec/cm5) | 327.5 ± 189.5 | 305.4 ± 201.1 | 0.242 |
| SBP (mmHg) | 139.9 ± 19.9 | 135.3 ± 21.8 | 0.247 |
| DBP (mmHg) | 82.8 ± 13.0 | 79.0 ± 9.3 | 0.085 |
| HR (bpm) | 75.3 ± 21.9 | 68.9 ± 14.7 | 0.043 |
Abbreviations: CI, cardiac index; DBP, diastolic blood pressure; HR, heart rate; mPAP, mean pulmonary arterial pressure; PCWP m, mean pulmonary capillary wedge pressure; NO, nitric oxide; PA d, diastolic pulmonary arterial pressure; PA s, systolic pulmonary arterial pressure; PVR, pulmonary vascular resistance; SBP, systolic blood pressure.
Figure 2.
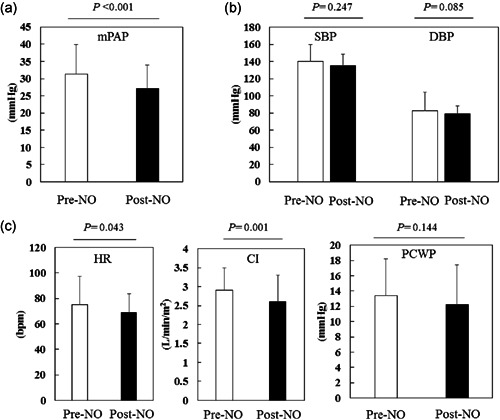
Hemodynamic changes by nitric oxide (NO) inhalation. (a) The mean pulmonary arterial pressure (mPAP) was significantly lowered by NO inhalation. (b) The systemic blood pressure (systolic and diastolic) did not decrease with NO inhalation. (c) The heart rate (HR) and cardiac index (CI) were significantly lowered by NO inhalation, but pulmonary capillary wedge pressure (PCWP) did not significantly change by NO inhalation
There were no significant differences in PVR (from 327.5 ± 189.5 to 305.4 ± 201.1 dyne/sec/cm5; p = 0.242) or PCWP (from 13.4 ± 4.8 to 12.2 ± 5.2 mmHg; p = 0.144).
BPA complications
The analysis of BPA procedure‐related complications is shown in Table 3. Procedure‐related complications were observed in 46 (13.6%) of the 338 sessions. Thirty‐one sessions were during the non‐NO era (11.7%/266 sessions), 15 sessions were during the NO era (20.8%/72 sessions), and there were no statistically significant differences (p = 0.053). The incidence of hemoptysis was significantly higher during the NO era than during the non‐NO era (20.8% vs. 10.9%; p = 0.031). In contrast, all deaths and the use of positive pressure ventilation (fatal procedure‐related complications) occurred during the non‐NO era (7 sessions; 2.6%), but there was no statistically significant difference compared to the NO era (0 sessions; 0.0%, p = 0.353).
Table 3.
BPA procedure‐related complications
| Total | Non‐NO era | NO era | p value | |
|---|---|---|---|---|
| BPA procedures (sessions) | 338 | 266 | 72 | |
| all complications (sessions and proportion) | 46 (13.6%) | 31 (11.7%) | 15 (20.8%) | 0.053 |
| death (sessions and proportion) | 1 (0.4%) | 1 (0.4%) | 0 (0.0%) | 1.000 |
| severe respiratory failure (sessions and proportion) | 7 (2.6%) | 7 (2.6%) | 0 (0.0%) | 0.353 |
| hemoptysis (sessions and proportion) | 44 (13.0%) | 29 (10.9%) | 15 (20.8%) | 0.031 |
Abbreviations: all complications, all procedure‐related complications (death, severe respiratory failure and hemoptysis); BPA, balloon pulmonary angioplasty; severe respiratory failure, hypoxia using positive ventilation.
The comparison between the recent one‐year period in the non‐NO era (from December 2018 to November 2019; the recent non‐NO era) versus the NO era (from December 2019 to November 2020) was shown in Table 4. There were no significant differences in all complications between the two groups. The incidence of hemoptysis was also not significantly different between the recent non‐NO era and the NO era (17.1% vs. 20.8%; p = 0.563). One severe procedure‐related complication using positive ventilation occurred in the recent non‐NO era (1.3% vs. 0.0%; p = 0.329).
Table 4.
BPA procedure‐related complications (recent non‐NO era vs. NO era)
| Total | Recent non‐NO era | NO era | p value | |
|---|---|---|---|---|
| BPA procedures (sessions) | 148 | 76 | 72 | |
| All complications (sessions and proportion) | 28 (18.9%) | 13 (17.1%) | 15 (20.8%) | 0.563 |
| Death (sessions and proportion) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Severe respiratory failure (sessions and proportion) | 1 (0.7%) | 1 (1.3%) | 0 (0.0%) | 0.329 |
| Hemoptysis (sessions and proportion) | 28 (17.1%) | 13 (17.1%) | 15 (20.8%) | 0.563 |
Abbreviations: all complications, all procedure‐related complications (death, severe respiratory failure, and hemoptysis); BPA, balloon pulmonary angioplasty; NO era, December 2019 to November 2020; recent non‐NO era, December 2018 to November 2019; severe respiratory failure, hypoxia using positive ventilation.
DISCUSSION
CTEPH patient prognosis has improved with the progression of the BPA procedure. 3 , 4 , 5 Furthermore, BPA for improving symptoms and QOL is becoming more important, and aggressive procedures for complete revascularization are increasingly being selected. 3 , 4 Compared to the past procedures, more distal lesions (in subsegmental or more distal pulmonary arteries) have become the target for BPA by technological progress and accumulated experience. 3 , 4 Most complications of BPA are caused by procedure‐related pulmonary bleeding; therefore, recent aggressive treatment may increase the incidence of complications. 9 , 10 In this study, even a very small amount of blood in the sputum was judged as hemoptysis. Therefore, the frequency of hemoptysis was relatively high. However, uncontrollable blood sputum did not occur in the NO era. To increase the safety of the procedures, preventing the aggravation of complications is important.
A previous study reported that high mPAP was associated with the incidence of severe complications and suggested that lowering mPAP during the BPA procedure might reduce severe complications. 11 We hypothesized that selective pulmonary vasodilators would lower mPAP during procedures and prevent procedure‐related complications from becoming more severe.
NO inhalation also acts as a pulmonary vasodilator in patients with CTEPH caused by organized thrombi. The previous studies 13 , 14 have shown that inhaled NO significantly lowers mPAP and PVR and significantly increases CI. CTEPH and pulmonary arterial hypertension are reported to share some histological and pathophysiological features, 15 which may be why CTEPH responds to acute vasodilators. Our results reconfirmed that inhaled NO lowered mPAP and indicated that NO inhalation lowered only pulmonary arterial pressure without decreasing systemic blood pressure, even when CTEPD patients were included (Figure 2). Systemic blood pressure is monitored to assess the general condition during treatment, but if it drops, BPA will be difficult to continue. Therefore, our results, which showed that NO inhalation did not lower systemic blood pressure, are important for BPA. Our results, in contrast to previous reports, 13 , 14 did not show a significant improvement in PVR and CI. This may be due to the inclusion of CTEPD patients and CTEPH patients whose hemodynamics became milder after several BPA treatments. In addition, the small number of cases may have influenced the lack of significance.
NO inhalation was suggested to dilate pulmonary arteries in CTEPH/CTEPD patients but not to increase PCWP. Pulmonary edema was not reported in this study, including in relatively elderly patients. In addition, no complications by NO inhalation were reported in this study, and NO inhalation for CTEPH/CTEPD patients was safe to use.
In our results, the incidence of controllable hemoptysis was significantly higher in the NO era than in the non‐NO era. The vasodilation caused by inhaled NO may have inhibited the vasoconstriction against bleeding. Furthermore, inhaled NO inhibits platelet aggregation, 16 which may have increased the frequency of hemoptysis. However, we performed more aggressive procedures (e.g., treating far distal lesions or using a retrograde approach) for complete revascularization in recent years. 17 When we compare the frequency of complications between the NO era (December 2019 to November 2020) and the recent non‐NO era (December 2018 to November 2019), there were no significant differences. The aggressive BPA procedures may have influenced the difference in frequency of hemoptysis.
In contrast, no fatal complications (death and severe respiratory failure) occurred in the NO era (0.0%; compared to 7 events [2.6%] in the non‐NO era) despite the high frequency of hemoptysis. One severe complication (hypoxia using pressure ventilation) also occurred in the recent non‐NO era. This finding may indicate that the benefit of NO inhalation in lowering mPAP outweighs the disadvantage of NO inhalation (inhibition of platelet aggregation and vasoconstriction against bleeding). However, our results did not indicate that NO inhalation significantly reduced the incidences of severe BPA complications. More cases and proper trials may be required to show significance.
Taking oral pulmonary vasodilators (i.e., endothelin receptor antagonist, phosphodiesterase‐5 inhibitor, soluble guanylate cyclase stimulant, and prostacyclin analog) before BPA may lower mPAP during the procedure and prevent aggravation of procedure‐related complications. However, this hypothesis is unconfirmed due to a lack of reports. Oral pulmonary vasodilators are expensive and introducing them to all pre‐BPA CTEPH patients is a significant medical financial burden. In addition, oral pulmonary vasodilators are not indicated for CTEPD patients. In contrast, NO is less expensive and therefore has a lower medical financial burden, and may be useful for safer BPA procedures.
This hypothesis‐generating pilot study had some major limitations. First, this study was a single‐center and nonrandomized trial, so more cases and research will be needed to establish a safer BPA procedure by NO inhalation. In fact, our results did not show a significant reduction of severe BPA complications by NO inhalation. However, this study is the first report to examine the hemodynamic changes and complications induced by NO inhalation during BPA. Second, because we separated the NO and non‐NO groups according to time period, the aggressiveness of the procedure (achievement of complete revascularization) was different. 17 , 18 We selected this method because this study focused on procedure‐related complications and emphasized consecutive patients and procedures. However, we performed more aggressive BPA for complete revascularization in recent years. Therefore, the aggressiveness of the procedures may have influenced the frequency of hemoptysis. Furthermore, NO inhalation may have led to more aggressive BPA procedures. Third, we could not determine whether the reason for the increase in controllable hemoptysis in the NO era was due to the disadvantages of inhaled NO or due to aggressive treatment. Proper trials may help elucidate the answer. Forth, the operator's learning curve may have impacted the incidences and severities of procedure‐related complications. Fifth, the hemodynamic status of included patients is relatively mild, which may have influenced the assessment of the hemodynamic changes by NO inhalation.
NO inhalation during BPA acts as a selective pulmonary vasodilator in CTEPH/CTEPD patients. In the patients who inhaled NO during BPA, the frequency of hemoptysis doubled, but it was controllable, and the incidence of fatal complications was 0.0%.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Toho University Ohashi Medical Center (H20001). Comprehensive agreement was obtained from all patients and opt‐out from the website of Toho University Ohashi Medical Center provided the target patients with the opportunity to refuse participation in the present study.
AUTHOR CONTRIBUTIONS
Manuscript writing: Shota Saito. Study design: Nobutaka Ikeda. Data interpretation: Raisuke Iijima. Study design, data interpretation: Hidehiko Hara. Final approval: Masato Nakamura.
ACKNOWLEDGMENTS
We received a medical writing grant from Mallinckrodt Pharma K.K.
Saito S, Ikeda N, Iijima R, Hara H, Nakamura M. Evaluation of the effect of nitric oxide inhalation in the patients with chronic thromboembolic pulmonary hypertension or pulmonary disease during balloon pulmonary angioplasty. Pulm Circ. 2022;12:e12032. 10.1002/pul2.12032
REFERENCES
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 2. Fukuda K, Date H, Doi S, Fukumoto Y, Fukushima N, Hatano M, Ito H, Kuwana M, Matsubara H, Momomura S, Nishimura M, Ogino H, Satoh T, Shimokawa H, Yamauchi‐Takihara K, Tatsumi K, Ishibashi‐Ueda H, Yamada N, Yoshida S, Abe K, Ogawa A, Ogo T, Kasai T, Kataoka M, Kawakami T, Kogaki S, Nakamura M, Nakayama T, Nishizaki M, Kimura T, Kuriyama T, Nakanishi N, Nakanishi T, Tsutsui H, on behalf of the Japanese Circulation Society and the Japanese Pulmonary Circulation and Pulmonary Hypertension Society Joint Working Group . Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017). Circ J. 2019;83:842–945. 10.1253/circj.CJ-66-0158 [DOI] [PubMed] [Google Scholar]
- 3. Ikeda N. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiovasc Interv Ther. 2020;35:130–41. 10.1007/s12928-019-00637-2 [DOI] [PubMed] [Google Scholar]
- 4. Kataoka M, Inami T, Kawakami T, Fukuda K, Satoh T. Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. JACC Cardiovasc Interv. 2019;12:1382–88. 10.1016/j.jcin.2019.01.237 [DOI] [PubMed] [Google Scholar]
- 5. Ogawa A, Satoh T, Fukuda T, Sugimura K, Fukumoto Y, Emoto N, Yamada N, Yao A, Ando M, Ogino H, Tanabe N, Tsujino I, Hanaoka M, Minatoya K, Ito H, Matsubara H. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: Results of a Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2017;10:10. 10.1161/CIRCOUTCOMES.117.004029 [DOI] [PubMed] [Google Scholar]
- 6. Ikeda N, Hatano M, Nagamatsu T, Nakamura M. Successful right heart remodelling and subsequent pregnancy in a patient with chronic thromboembolic pulmonary hypertension undergoing balloon pulmonary angioplasty: a case report. Eur Heart J Case Rep. 2019;3:3. 10.1093/ehjcr/ytz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minatsuki S, Kodera S, Kiyosue A, Saito A, Maki H, Hatano M, Takimoto E, Komuro I. Balloon pulmonary angioplasty improves quality of life in Japanese patients with chronic thromboembolic pulmonary hypertension. J Cardiol. 2020;76:205–10. 10.1016/j.jjcc.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 8. Inami T, Kataoka M, Kikuchi H, Goda A, Satoh T. Balloon pulmonary angioplasty for symptomatic chronic thromboembolic disease without pulmonary hypertension at rest. Int J Cardiol. 2019;289:116–118. 10.1016/j.ijcard.2019.04.080 [DOI] [PubMed] [Google Scholar]
- 9. Ikeda N, Kubota S, Okazaki T, Iijima R, Hara H, Hiroi Y, Nakamura M. The predictors of complications in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Catheter Cardiovasc Interv. 2019;93:E349–56. 10.1002/ccd.28133 [DOI] [PubMed] [Google Scholar]
- 10. Kawakami T, Ogawa A, Miyaji K, Mizoguchi H, Shimokawahara H, Naito T, Oka T, Yunoki K, Munemasa M, Matsubara H. Novel angiographic classification of each vascular lesion in chronic thromboembolic pulmonary hypertension based on selective angiogram and results of balloon pulmonary angioplasty. Circ Cardiovasc Interv. 2016;9:9. 10.1161/CIRCINTERVENTIONS.115.003318 [DOI] [PubMed] [Google Scholar]
- 11. Ejiri K, Ogawa A, Fujii S, Ito H, Matsubara H. Vascular injury is a major cause of lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2018;11:e005884. 10.1161/CIRCINTERVENTIONS.117.005884 [DOI] [PubMed] [Google Scholar]
- 12. Ichinose F, Roberts JD, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–11. 10.1161/01.CIR.0000134595.80170.62 [DOI] [PubMed] [Google Scholar]
- 13. Ulrich S, Fischler M, Speich R, Popov V, Maggiorini M. Chronic thromboembolic and pulmonary arterial hypertension share acute vasoreactivity properties. Chest. 2006;130:841–846. 10.1378/chest.130.3.841 [DOI] [PubMed] [Google Scholar]
- 14. Skoro‐Sajer N, Hack N, Sadushi‐Koliçi R, Bonderman D, Jakowitsch J, Klepetko W, Hoda MA, Kneussl MP, Fedullo P, Lang IM. Pulmonary vascular reactivity and prognosis in patients with chronic thromboembolic pulmonary hypertension: a pilot study. Circulation. 2009;119:298–305. 10.1161/CIRCULATIONAHA.108.794610 [DOI] [PubMed] [Google Scholar]
- 15. Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest. 1993;103:685–92. 10.1378/chest.103.3.685 [DOI] [PubMed] [Google Scholar]
- 16. Samama CM, Diaby M, Fellahi JL, Mdhafar A, Eyraud D, Arock M, Guillosson JJ, Coriat P, Rouby JJ. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1995;83:56–65. 10.1097/00000542-199507000-00007 [DOI] [PubMed] [Google Scholar]
- 17. Toi S, Ikeda N, Iijima R, Hara H, Nakamura M. Successful transcollateral bidirectional balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv. 2022;14(e281‐e282):20210929. 10.1016/j.jcin.2021.07.049 [DOI] [PubMed] [Google Scholar]
- 18. Saito S, Ikeda N, Toi S, Nakamura M. Gadolinium contrast balloon pulmonary angioplasty for a patient with chronic thromboembolic pulmonary hypertension and severe iodine allergy. Catheter Cardiovasc Interv. 2020;97:525. 10.1002/ccd.29004 [DOI] [PubMed] [Google Scholar]


