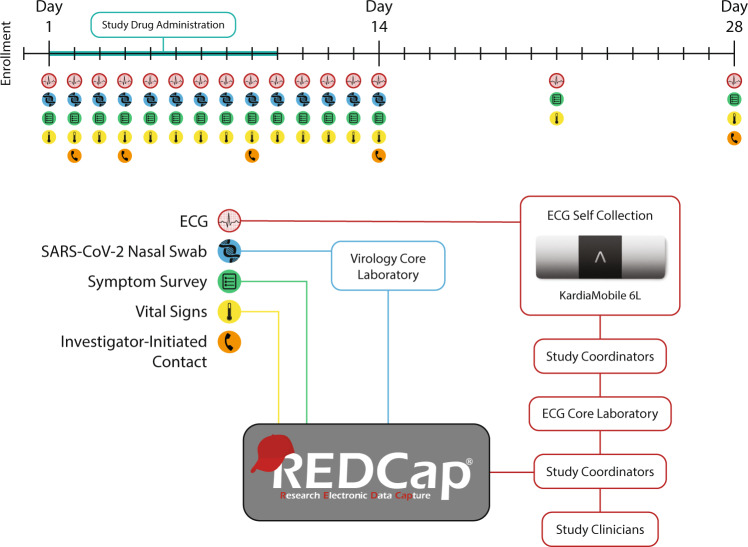
Fig. 1. Timeline of participant study drug administration and data collection with schematic demonstrating data processing and storage.
Visual representation of the study protocol. Study drugs were taken on protocol days 1–10. ECGs, mid-nasal viral swabs, symptom surveys, and vital signs were self-collected on protocol days 1–14, while only ECGs, symptom surveys, and vital signs were obtained on days 21 and 28. On days 2, 4, 9, 14, and 28, planned investigator-initiated contact was undertaken to collect subjective data and encourage adherence. Vital signs were collected twice daily. ECGs were uploaded to the secure web portal where study coordinators pushed them to the core ECG laboratory and then recorded the returned interpretation, as described in the “Methods” section. Viral swabs were sent to the core virology laboratory. All data were warehoused in REDCap33.