Figure 1: Pain pathway and sites of inflammation and neuroplasticity in osteoarthritis.
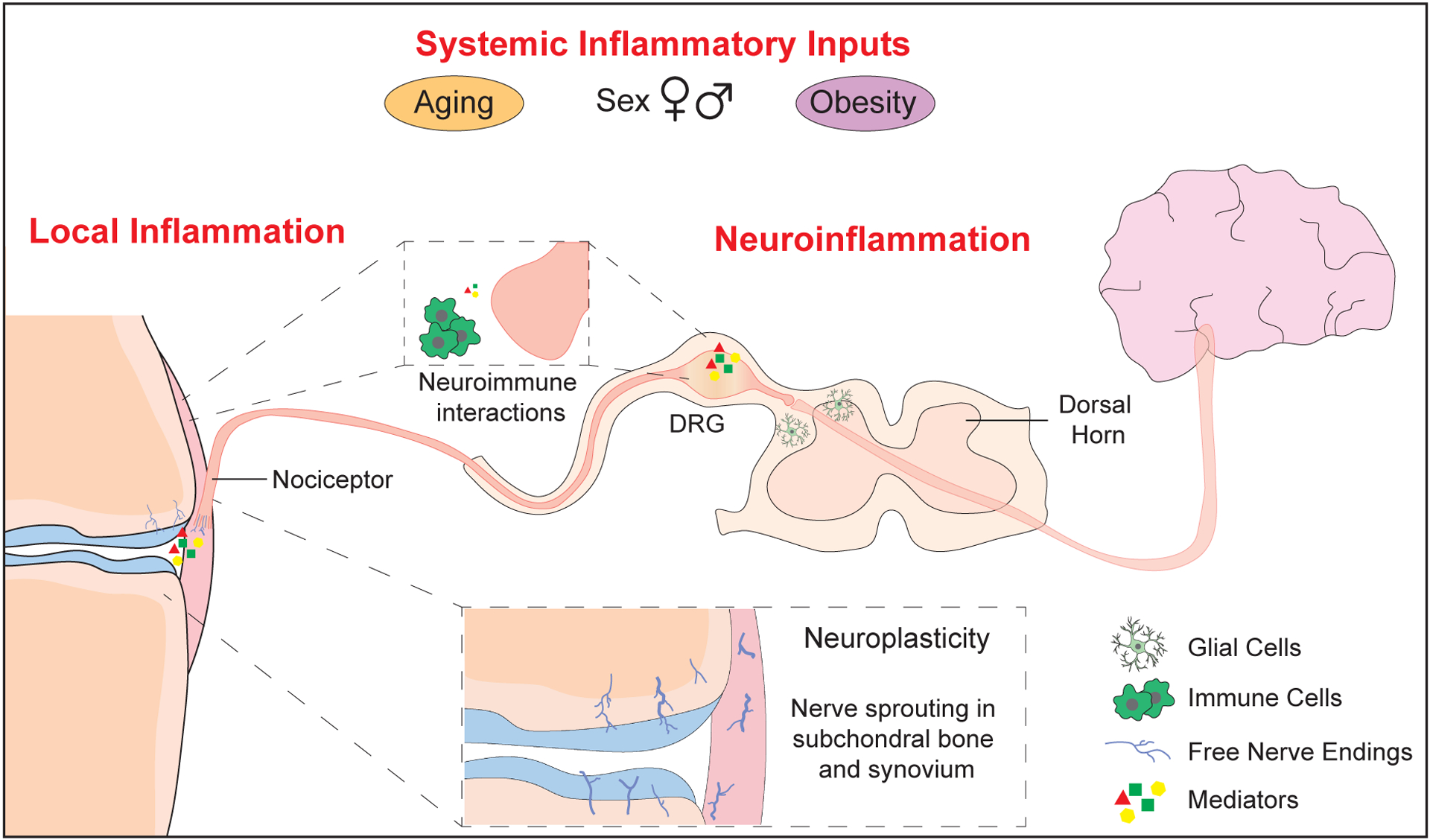
Nociceptors (Aδ or C- fibers) detect stimuli in joint tissues, generating action potentials that are transmitted to the dorsal horn via the dorsal root ganglia (DRG), and to the brain via the central nervous system. Inflammatory states and neuroimmune interactions can occur at multiple points along this pathway. Structural neuroplasticity occurs in the OA joint, with free nerve endings sprouting in the subchondral bone and synovium. Joint nociceptors become sensitized by mediators released in OA pathology, including nerve growth factor (NGF), inflammatory cytokines and chemokines, and damage-associated molecular patterns (DAMPs). Immune cell infiltration in synovium and DRG can include macrophages, T- and B- lymphocytes, and mast cells, which can further amplify pain mechanisms. Neuroimmune interactions also occur in the DRG and the dorsal horn. Finally, factors such as obesity, age, and sex may alter systemic inflammatory inputs and modify OA pain.
