Abstract
Aims:
To characterize exercise fatigue, metabolic phenotype and cognitive and mood deficits correlated with brain neuroinflammatory and gut microbiome changes in a chronic Gulf War Illness (GWI) mouse model. The latter have been described in an accompanying paper [1].
Main methods:
Adult male C57Bl/6N mice were exposed for 28 days (5 days/week) to pyridostigmine bromide: 6.5 mg/kg, b.i.d., P.O. (GW1) or 8.7 mg/kg, q.d., P.O. (GW2); topical permethrin (1.3 mg/kg in 100% DMSO) and N,N-diethyl-meta-toluamide (DEET 33% in 70% EtOH) and restraint stress (5 min). Exercise, metabolic and behavioral endpoints were compared to sham stress control (CON/S).
Key findings:
Relative to CON/S, GW2 presented persistent exercise intolerance (through post-treatment (PT) day 161), deficient associative learning/memory, and transient insulin insensitivity. In contrast to GW2, GW1 showed deficient long-term object recognition memory, milder associative learning/memory deficit, and behavioral despair.
Significance:
Our findings demonstrate that GW chemicals dose-dependently determine the presentation of exercise fatigue and severity/type of cognitive/mood-deficient phenotypes that show persistence. Our comprehensive mouse model of GWI recapitulates the major multiple symptom domains characterizing GWI, including fatigue and cognitive impairment that can be used to more efficiently develop diagnostic tests and curative treatments for ill Gulf War veterans.
Keywords: Central nervous system, Insulin insensitivity, Novel object recognition memory, Passive avoidance learning, Diabetes, Mood, Depression, Cognitive impairment
1. Introduction
Gulf War Illness (GWI) is defined as a chronic multi-symptom disorder that still affects approximately one-third of US veterans that served in the 1990–1991 Persian Gulf War (GW) [2]. GWI is characterized by a constellation of latently-emerging and persistent symptoms that, according to the Kansas case definition, fall into 6 general domains: neurological/cognitive/mood, muscle/joint pain, skin, respiratory, gastrointestinal disturbance and especially chronic fatigue [3–6]. The pathophysiology of GWI has not been established and there are still no curative treatments available in spite of 30 years of debilitating conditions that are now further exacerbated by aging factors.
A leading hypothesis for the etiology of GWI is the association of physical and psychological stress of combat, in combination with exposure to Gulf War-related chemicals administered prophylactically [7], such as the insecticide permethrin (PER) and the insect repellant, N, N-diethyl-meta-toluamide (DEET) and especially orally-administered pyridostigmine bromide (PB), a reversible acetylcholinesterase inhibitor (AChEI), that protects against the harmful effects of nerve agents [8]. Although these chemicals were administered to GW personnel at doses considered safe [7], subsequent animal research suggests that they synergistically contribute to GWI symptomology [7,9–11]. Multiple lines of evidence, including the multi-symptom nature of GWI, converge on the idea that the underlying pathobiology likely includes the central nervous system (CNS), corroborated by neuroimaging and biomarker studies [7,12]. After exposure to stress and GW-related chemicals, the blood brain barrier may become leaky, allowing PB to target central components of the cholinergic system [10,13], which may include basal forebrain cholinergic neurons, whose projections to the cortex are necessary for memory, attention and sleep [14,15], domains all altered in GWI.
Chronic fatigue (CF) and cognitive deficits are two of the most widely reported symptoms in Gulf War veterans (GWVs) [16,17]. GWVs complain of debilitating CF symptoms defined as a subjective lack of physical and/or mental energy perceived to significantly interfere with daily activities that dramatically impairs quality of life [18]. Importantly, long-term studies have suggested that symptoms do not improve and, instead, worsen over time in this aging population [19,20]. The widespread and multi-symptom characteristics of GWI, in combination with neuropathological correlates, may indicate centrally-mediated cascades that influence multiple physiological outcomes. Disturbingly, few studies have been conducted to elucidate the biological mechanisms underlying chronic fatigue in GWI veterans or experimental animal models. The fatigue phenotype of GW mice is especially poorly understood because of the lack of a suitable model, specifically one that also recapitulates GWI cognitive parameters. Moreover, fatigue and cognitive impairment have been associated with diabetes [21], suggesting that glucose dyshomeostasis may be comorbid with GWI manifestations. Recent reports show higher incidence of diabetes and other chronic diseases associated with aging in the GWV population [4,20,22].
In this study we have characterized the cognitive, fatigue and metabolic phenotypes produced by chronic exposure to the GW agents PER, DEET and PB at one of two doses (GW1, GW2) using behavior, exercise endurance and insulin tolerance parameters and compared those phenotypes to those manifested in stress- and vehicle-exposed sham controls. To be relevant to the delayed/persistent pathophysiology in GWVs, analysis was performed at delayed time points ranging from 3 weeks to 6.6 months after GW agent exposure. Our findings characterize a comprehensive mouse model of GWI displaying symptoms associated with neurological/cognitive/mood and fatigue categories used by the Kansas case definition [3]. Specifically, in GW2 we demonstrate, for the first time, exaggerated exercise fatigue, reduced passive avoidance learning and memory and transient glucose dyshomeostasis. In contrast, GW1 showed deficient long-term novel object recognition memory, less severe passive avoidance deficit, concomitant with behavioral despair. Our results indicate that GW1 and GW2 did not display a common phenotype, indicating that the PB dose may dictate the occurrence and characteristics of GWI phenotypes. Tissues from these animals were used to determine neuromolecular signatures and other biomarkers associated with these GW phenotypes described in the accompanying article in this issue [1]. Our comprehensive mouse model provides an integrated substrate for diagnosis of multiple symptoms established and emerging in GWI, for examination of underlying pathophysiology as well as for testing potential targets for therapeutic benefit.
2. Materials and methods
2.1. Animal care and maintenance
C57Bl/6N mice were generated using breeders originally obtained from Charles River Labs (West Sacramento, CA) or Taconic (Germantown, NY). Mice were housed 2–4 per cage in standard static polycarbonate plastic cages with corn-cob bedding containing one cotton Nestlet square for enrichment in a specific pathogen-free vivarium and kept on a 12:12-h light:dark cycle, in a controlled temperature (21.1–22.8 °C) environment. Relative humidity ranged between 20 and 70%. Mice were provided rodent chow (LabDiet 5001; Laboratory Diets, USA) and water ad libitum. Care and treatment of animals was performed in compliance with guidelines from and approved by the University of California Riverside Institutional Animal Care and Use Committee (AUP#20210024, 20200020 and 20180067).
2.2. GW agent exposure paradigm
GW agents used consisted of N,N-diethyl-meta-toluamide (DEET) (100 Maximum Formula, Coleman), which contains 98.1% DEET, permethrin (PER) (Crescent Chemical Co., Inc.), and pyridostigmine bromide (PB; TLC Pharmaceutical Standards). A chronic exposure paradigm was used as described for other GWI models [10,23]. These models used routes of administration that are relevant to GW veterans since PB was taken orally and PER and DEET exposure occurred through skin and inhalation [4,5,24]. At 7–10 weeks of age mice were randomly assigned to GW-exposed or sham-treated control groups. Animals were exposed to PB via oral gavage whereas PER and DEET were applied dermally to a shaved region on the back of the neck. Group GW1 received PB at the dose of 6.5 mg/kg (b.i.d., P.O.), while group GW2 received 8.7 mg/kg (q.d., P.O.). Exposures were dispensed in 5-day periods separated by 2-day rest for a duration of 28 days. Both groups received 1.3 mg/kg PER in 100% DMSO (75 μl/30 g bw; topical) and 33% DEET in 70% EtOH (75 μl/30 g bw; topical). We included 2 groups to control for sham (CON) and for sham/stress (CON/S) treatment. Shams received 0.9% saline (150 μl/30 g, P.O. b.i.d) and 70% ETOH (75 μl/30 g bw, topical) and 100% DMSO (75 μl/30 g bw, topical). The CON/S group also received 5 min restraint stress in perforated 50 mL conicals once daily [25]. Animals were tested on metabolic, behavior and exercise parameters as shown (Fig. 1). After mice were anesthetized and sacrificed at PT199, tissues were harvested and analyzed as shown in the accompanying article [1].
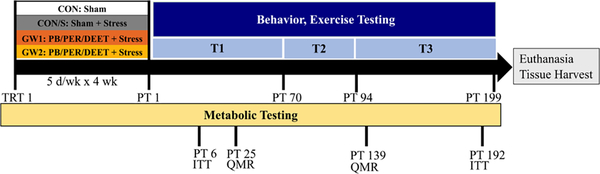
Fig. 1.
GW exposure model and endpoints. Four experimental groups of adult mice were used: a sham treated control (CON), a sham treated control that received restraint stress (CON/S), and two GW groups that received one of two doses of PB P.O.: 6.5 mg/kg b.i.d. (GW1) and 8.7 mg/kg (GW2), plus topical DEET and PER as well as 5 min restraint stress. The exposure regimen was given 5 days/week followed by 2 day rest for 4 weeks. Animals were tested on metabolic, behavior and exercise endpoints as described. We tested the persistence of phenotype by testing for selected behaviors at various post-treatment time points T1 (PT 25–70), T2 (PT 70–94), T3 (94–199). Metabolic endpoints assessed include insulin sensitivity (IP ITT), post-exercise blood lactate and glucose, body weight and composition (QMR). Behavior tests included: SUOK, elevated plus maze (EPM), forced swim test (FST), tail suspension test (TST), novel object recognition test (NORT), sucrose splash test (SST), passive avoidance test (PAT), exercise endurance (EE), Rotarod, Barnes maze (BM), hot plate test (HPT). Mice were tested initially (1×) and repeat-tested on selected behaviors once (2×) and twice (3×) to determine onset and persistence of latently-emerging GW pathology as described in captions of figures below. DEET, N,N-diethyl-meta-toluamide; PB, pyridostigmine bromide; PER, permethrin; PT, post-treatment; QMR, quantitative magnetic resonance; T, time point; TRT, treatment; GW1, 6.5 mg/kg b.i.d.; GW2, 8.7 mg/kg.
PB dose was determined based on 90 mg/day taken by troops, i.e., 30 mg/pill × 3 pills/day [26]. Based on a male body weight of 75 kg the dose is estimated at 90 mg/75 kg or 1.2 mg/kg. To convert the human equivalent dose (HED) to the animal dose we examined several methods including the use of a scaling factor to account for body surface area and metabolic rate as described [27]. Using the equation HED (mg /kg) = Animal dose (mg /kg) X Km ratio one calculates a correction factor (Km) that is estimated by dividing the average body weight (kg) of species to its body surface area (human − 1.62 m2 and mouse − 0.007 m2). Assuming 75 and 0.025 kg bw for human and mouse, respectively, correction factors are 46.3 and 3.6, respectively. The Km ratio is obtained by dividing animal Km by human Km constants or 0.077. Animal dose was calculated as the human dose 1.2 mg/kg divided by Km ratio or 15.5 mg/kg, which was near the LD50 for PB in mice fed through the oral route (Cayman Chemical). In pilot experiments, mice exhibited symptoms of acute PB toxicity due to overstimulation of the PNS at muscarinic and nicotinic sites, i.e., tremors, labored breathing and lacrimation. Therefore, the PB dose was given twice daily at roughly half the dose (6.5 mg/kg) for GW1.
We followed the US Environmental Protection Agency (EPA) recommended use of body weight (BW) ¾ scaling [28] to derive the 8.7 mg/kg PB dose. The BW Scaling Factor was found by first dividing the human male body weight (75 kg) by the average weight of a mouse (0.03 kg). This value was then raised to ¾, the recommended scaling value:. The 1.2 mg/kg dose of PB taken by troops was then multiplied by the average male body weight, and this value was divided by the BW scaling factor to find the dose for mice: (1.2 mg/kg × 75 kg) / 354 = 0.261 mg. This value divided by the average weight of a mouse determined the PB dosage given to GW2 (8.7 mg/kg).
2.3. Body composition
Whole body composition was determined in live, unanesthetized mice by use of quantitative magnetic resonance (QMR) system, which relies on nuclear magnetic resonance (NMR) technology (EchoMRI; Echo Medical Systems, Houston, TX) [29]. Using various pulse sequences, the QMR system provides estimates of fat mass, lean tissue mass, free water, and total body water. Briefly, this system generates a signal that modifies the spin patterns of hydrogen atoms within the subject, and uses an algorithm to evaluate the individually derived T1 and T2 relaxation curves fractionated from the total returned signal specific to fat mass, lean muscle mass equivalent, and free water. This QMR system has been demonstrated comparable to whole-body chemical carcass composition analysis, but with higher overall precision. In addition, it can quantify differences in mice subjected to 18 h water and food deprivation protocols [30]. In brief, duplicate QMR scans were performed by placing previously-weighed mice into a well-ventilated plastic cylinder (1.5 mm thick, 4.7 cm inner diameter), with a cylindrical plastic insert added to limit movement. While in the tube, animals were briefly subjected to a low-intensity (0.05 Tesla) electromagnetic field. Duplicate QMR scans were performed with accumulation times of 2 min to determine mean fat and lean mass, expressed at % body weight.
2.4. Insulin tolerance testing
An insulin tolerance test (IP ITT) was performed to assess glucose homeostasis by evaluating the effectiveness of the clearance of exogenous insulin load administered by intraperitoneal (ip) injection. At 6 days post cessation of GW agent exposure, mice were administered 0.5 U/kg Humulin N ip (Eli Lilly, USA) in sterile saline (0.9% NaCl) after a light phase fast of 7.5 h. In another group of mice, ITT was conducted at 192 days post GW treatment. Mice were administered 1.0 U/kg Humulin R ip after a 5 h light phase fast. Tail blood glucose was measured before and at 15, 30, 45, 60, 90 and 120 min after insulin bolus. Tail blood glucose concentrations were measured with a glucometer (One Touch Ultra 2, Lifescan Inc., USA) and test strips, shown to be accurate in C57Bl/6 mice [31]. To determine insulin sensitivity, the blood glucose reduction rate after insulin administration, KITT, was calculated over the initial slope of the ITT glucose response curve using the formula 0.693 × t1/2. Half-life (t1/2) was calculated from the slope of the blood glucose concentration [23] from 0 to 30 min post-insulin injection, when plasma glucose concentration declines linearly in response to insulin and represents insulin sensitivity [32–34]. The inverse area (AUC) under the percent basal ITT glucose curve was calculated using Prism (GraphPad). In brief, the definite integral of the AUCITTglucose was geometrically approximated using the trapezoid rule as the area of time units (x) multiplied by glucose units (y). The inverse AUC for each animal was obtained by subtracting the calculated AUC from the total possible area defined by x and y units or 12,000%*min (100% baseline * 120 min).
2.5. Neurobehavioral testing
At least 30 min prior to testing, mice were moved to a designated behavior room. Ethanol (70%) was used to remove debris and odors on apparatus between individual mouse trials. Unless stated otherwise, mouse behavior was recorded using digital cameras driven by Logitech software and scored a posteriori using Ethovision (Noldus), ANY-Maze (Stoelting Co.) or manually using BORIS [35] performed blind to treatment by a trained observer. The passive avoidance, sucrose splash and the forced swim tests were scored manually with a digital timer. Mice were tested during the light phase of a 12:12 h light/dark cycle (lights on at 0700 h). To reduce cross-over effects, behavioral tests were performed after at least 1 day rest. Certain tests were repeated at different post-treatment days (Fig. 1).
2.6. Exercise endurance (EE)
Latency to exhaustion was measured using a previously published protocol for exercise endurance in mice [36]. A human treadmill was modified to accommodate mice as described [37]. Briefly, a frame with four tracks, each 92 cm × 8 cm × 16 cm, was overlaid to define the narrow running area for each test mouse. The treadmill incline was set to a 0° incline. Mice learned to avoid sharp bristle brushes placed inside and at the end of each track. Mice were subjected to 3 days of training followed by 2 days of testing. During training, mice ran for 15 min at increasing speeds of 10, 14, and 18 m/min, respectively. Animals not able to successfully complete training were excluded from testing. During testing, the treadmill was set to 20 m/min and the speed was increased by 3 m/min at 4 min intervals. Testing was completed when the subject hit brush 4 times in a span of 10 s, the predetermined criterion for exhaustion. Mice that stumbled on the brush prior to meeting the exhaustion criterion were placed at the front of the treadmill to resume testing. One day prior to training, tail blood was sampled to obtain baseline glucose and lactate values (Accutrend meter). Post-testing glucose and lactate values were obtained on the last testing day immediately following test completion.
2.7. SUOK
SUOK is an elevated platform behavioral paradigm used as an experimental tool to analyze anxiety, motor-vestibular anomalies, as well as anxiety-induced motor impairments in mice [38]. The SUOK apparatus consists of a smooth (slippery) aluminum rod (2 m long, 3 cm diameter) elevated to a height of 20 cm and fixed to two clear acrylic vertical walls [38]. After acclimation to the dimly lit testing room for 30–60 min, several behaviors were scored over a 5 min trial as the animal walked along the aluminum rod: (1) horizontal and locomotor (normalized to active time) activity, assessed as number of segments travelled, (2) sensorimotor coordination represented as the number of hind leg slips and falls from the rod, (3) exploratory behavior such as side looks and head dips, (4) anxiogenic behaviors such as increased latency to leave the central zone, and unprotected (over the borders of rod) stretch-attend postures (SAP) in which the mouse stretches forward and retracts without moving its feet, [39,40], (5) vegetative responses (combined number of urinations and defecation boli), and (6) auto-grooming behaviors.
2.8. Accelerated rotarod test
The rotarod test was adapted from [41]. Motor ability, learning, and coordination were tested using an accelerated Rotarod apparatus (Ugo Basile; [42,43]) placed on a table top. Animals were placed on a rotating bar that accelerates from 4 to 40 rpm/min for 5-minute trials. Each rotarod test consisted of 4 trials with a 10 min inter-trial interval. Latency to fall was measured within each trial. If the animal was successful in maintaining its position on the rotating rod without falling for the entire duration of the trial, a maximum score of 5 min was assigned.
2.9. Step-through passive avoidance test (PAT)
Mice were subjected to an 8 day passive avoidance task (PAT) procedure to assess impairments in avoidance learning and emotional memory processing [44]. The task requires the animal to associate a neutral environment with an aversive stimulus and is hippocampal function-dependent [45,46]. During acquisition, mice were placed in the brightly lit (1800 lux) chamber of a light-dark box with access to the dark chamber; the door between chambers was kept open. The cross-over latency to enter the dark chamber, the naturally preferred context during acquisition, was recorded. On entering the dark compartment, the door was immediately closed and the mouse received a single mild (1 mA, 3 s) electric foot shock (Unconditioned stimulus, UCS) delivered via stimulation pads (LG Med Supply, USA). Mice were allowed to remain in the dark chamber for 10 s after the foot shock to associate the environment with the aversive stimulus. During subsequent tests performed at 2, 4 and 8 days, mice were placed into the bright compartment and latency scores recorded with a predetermined cut-off criterion (300 s without crossover). Once crossing over or reaching criterion, mice were removed from the lit chamber (no foot-shock given). Passive avoidance learning was assessed as an increased latency to enter the aversive context by learning to associate the context with the UCS.
2.10. Hot plate test (HPT)
The hot plate test measures an animal’s tolerance to a thermal stimulus and, therefore, informs about pain threshold [47]. A cast iron steel pan (1.9 cm thick) was placed over a hot plate on a water bath. The surface temperature was maintained at 53 ± 0.5 °C as measured by an infrared thermometer (General IRT-207). The latency of the animal to exhibit nociceptive behavior (i.e. hindpaw withdrawal or licking, jumping) was recorded [48]. A predetermined cut-off time after which the test was stopped was set at 30 s to prevent tissue damage.
2.11. Novel object recognition test (NORT)
The novel object recognition test (NORT) was used to assess short and long-term object recognition memory [49] with modification as described [50]. Intrinsic preference for object pairs was ruled out in optimization trials [51]. First, the test mouse was habituated to a square plexiglass open field (40 × 40 × 40 cm) for 10 min in the absence of objects as described. The test mouse was then returned to their home cage for 20 min. During the acquisition phase, the test mouse was placed in the experimental apparatus containing two identical objects placed in adjacent corners, and allowed to freely explore the environment and objects for 5 min. During the short-term memory testing session, the test mouse was again placed in the apparatus after a retention time of 30 min, where this time one of the familiar objects (F) was replaced by a novel one (N), and allowed to explore for 5 min. After a 24 h retention time, long-term memory was assessed by placing mice into the apparatus with the familiar (F) and second novel object (N’). It should be noted that in the NORT paradigm adapted for 2 testing days the same mice were used for both 30 min and 24 h retention periods as described [50,52] and, therefore, performance on the 24 h retention test cannot be completely separated from likely carryover effects of the 30 min test. Preference for the novel object was expressed using a discrimination index, calculated as the difference of time exploring novel relative to total time exploring both objects, where 0 indicates no preference for either object. We applied an exclusion criterion if the test mouse did not visit the familiar or novel target zone at least 6 times, which we determined was required to confirm the construct validity of the test, i.e. that mice spent sufficient time with objects in order to learn/discriminate them. If a subject failed to meet this criterion on any phase (training, 30 min or 24 h testing) then it was excluded from the data for all trials.
2.12. Open field test (OFT)
The open field test allows rapid assessment of rodent locomotion, anxiety and habituation without a training requirement [53]. The open field apparatus, a square arena of 40 × 40 cm (1600 cm2 of floor space) enclosed by continuous 40-cm-high walls made of clear plexiglass, is a large, brightly lit and aversive environment for rodents. Locomotor and other activity was examined over a 10 min period to determine distance travelled, velocity and total time in periphery (10 cm adjacent to wall) and center.
2.13. Barnes maze
The Barnes maze [54] was used to assess spatial and learning memory impairments. The Barnes maze is based on the assumption that the animal placed onto the surface of a platform should learn and remember the location of an escape box by use of distant visual spatial cues hung on the 4 surrounding walls. An extended version of the protocol [55,56] was used to test different aspects of learning such as reference memory (short and long-term) and to probe for cognitive flexibility through the implementation of reversal learning trials. The protocol involved 5 stages: habituation (day 0), acquisition trials (days 1–5), acquisition probe trial (24 h, 7d), reversal trials (occurred following 7 day acquisition probe; days 1–4), and reversal probe trial (24 h). At the beginning of each acquisition trial, the animal was placed in the middle of the maze. If the animal did not enter the escape hole after 3 min, it was gently guided to the escape box and allowed to stay there for 30 s before being returned to the home cage. During the acquisition probe trials a decoy box replaced the escape box and the apparatus was rotated 180° to avoid odor cues. During the reversal learning trials, the escape chamber was placed 180° from the previous location and probe trials conducted similarly. Both the time spent and latency to enter the escape or decoy holes in respective trials were measured. The trials were scored with ANY-Maze (Stoelting Co.) software and confirmed by manual scoring.
2.14. Forced swim test (FST)
The forced swim test (FST) was used to assess despair behavior. The test was performed as described [57]. Mice were individually placed in a cylinder of water (20 cm in diameter and 30 cm height), filled with 15 cm of water (25 ± 1 °C), for 6 min. Water was changed between each test animal. After the test, mice were removed and dried before returning to their home cage. The test was recorded and the immobility behavior was analyzed during the last 4 min. Mice were judged to be immobile when making no or only movements necessary to keep their head above water.
2.15. Sucrose splash test (SST)
To measure self-care behavior as an indication of depressive-like behavior, mice were subjected to a sucrose splash test (SST) as previously described [58]. Briefly, mice were placed individually in a clear observation cage without food and the dorsal coat sprayed with a 10% sucrose solution. The sucrose solution dirties the mouse fur and elicits grooming behavior. The latency to the first grooming and duration was recorded during a 5-min trial. Depressive-like behavior has been shown to decrease the duration of grooming [59].
2.16. Tail suspension test (TST)
The tail suspension test (TST) was used as an additional measure of depressive-like behavior. TST was performed as described [60]. Briefly, mice were suspended on the edge of a table (elevated ~60 cm above the ground) using tape that was attached 2 cm from the tip of their tails. Tail climbing was prevented by passing the mouse’s tail through a small plastic cylinder prior to suspension. The duration of immobility was recorded and analyzed over a 6-min period. The animals were considered immobile when they hung down passively and remained completely motionless. Test mice were assigned a score based on the average of 3 trials conducted with an inter-trial interval of 20 min.
2.17. Elevated plus maze (EPM)
Anxiety-like behavior was assessed using the elevated plus maze (EPM) consisting of 2 open and 2 closed arms (20 × 22 cm) elevated 92 cm and constructed from black plexiglass as described [61]. The lighting was adjusted to create anxiogenic conditions, i.e., so that the open and closed arms received differential lighting, i.e., 300 and 30 lux, respectively. At the beginning of each trial, the test mouse was placed in the center zone facing the open arm and was allowed to explore the apparatus for a total of 5 min. Activity was monitored by an overhead camera (LogiTech) and recordings were later analyzed for time spent in each arm.
2.18. Statistical analysis
Statistical analysis was performed using GraphPad Prism (v8.4.3 San Diego, CA, USA), unless otherwise specified. The normality of data distribution was evaluated with Shapiro-Wilk test and the equality of variances across groups was compared using F-test. For parametric data, unpaired one-sample t-test, two-tailed paired Student’s t-test, one-way ANOVA or RM-One-way ANOVA followed by Tukey’s or Dunnet’s post hoc or RM Two-way ANOVA or Mixed-Effects model with Tukey’s or Sidak’s post hoc tests were used to compare means within and across groups where appropriate. A Brown-Forsythe ANOVA was used instead if the group variances were significantly different. Biological outliers were excluded when values exceeded 2 × standard deviations, 2 × s.e.m. from the mean or using ROUT test (Q = 5%). For nonparametric data, a Kruskal-Wallis H test with Dunn’s post hoc was used to compare differences across groups. Type 1 error rate (α) was set at 0.05; F and p values are presented in the figure legends or Supplementary Information 1, Statistical Results.
3. Results
3.1. GW2 produces transient insulin insensitivity without altering body composition
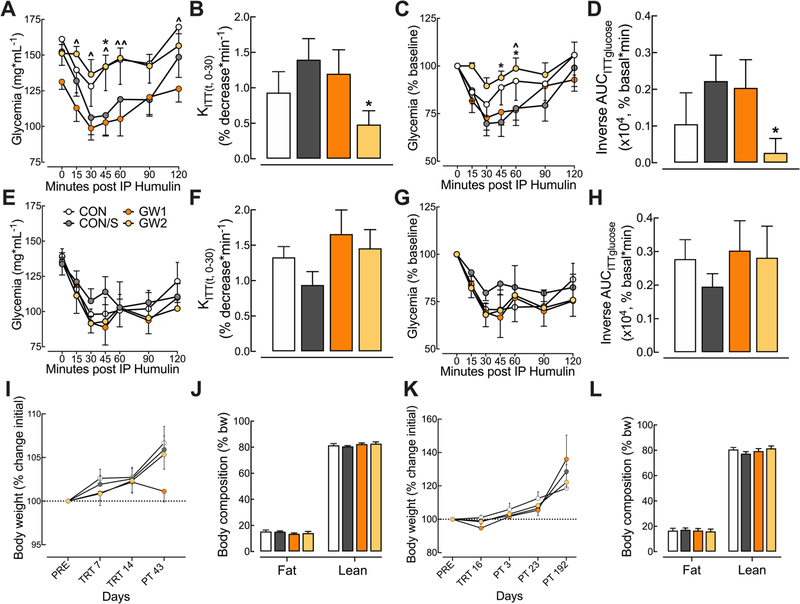
Six days after the end of the GW exposure period, the glycemia response to exogenous insulin was assessed during IP ITT experiments at PT 6. The mean glycemia values over the 120 min time-course following insulin injection are shown in the insulin tolerance curve (Fig. 2A). GW2 (but not GW1) displayed less reduction in glycemia relative to CON/S at t = 45 (p < .05). GW2 mice also displayed less reduction in glycemia as compared to GW1 at several time points post-injection (t = 15, 30, 45, 60 and 120; p < .05–.01). Since the glycemia response over the 120 min observation period is due to complex actions of insulin, we measured insulin sensitivity by calculating the rate constant for glucose reduction (KITT) over the first 30 min post-injection (Fig. 2B). This metric showed a significant decrease in the plasma glucose disappearance rate for GW2 relative to CON/S (p < .05). The absolute glycemia values were not different at t = 0, therefore, glycemia was expressed as percent of baseline (Fig. 2C) [34]. Similarly, the GW2 group displayed less post-insulin reduction in glycemia relative to CON/S at t = 45 and 60. The corresponding inverse AUCITTglucose of the percent baseline curve showed that GW2 had significantly less reduction in glycemia over the 120 min time-course (p < .05; Fig. 2D). In contrast, there were no differences between groups in a second ITT conducted at PT192 for absolute glycemia values (Fig. 2E), KITT (Fig. 2F), glycemia expressed as percent baseline (Fig. 2G) nor corresponding inverse AUCITTglucose (Fig. 2H) suggesting a transient effect of GW2 on insulin response.
Fig. 2.
GW2 produces transient insulin insensitivity without affecting body composition. (A–H) Mice were subjected to an IP ITT test at PT 6 and 192. (A) Absolute blood glucose concentrations measured before and at t = 15, 30, 45, 60, 90 and 120 min post-insulin injection during IP ITT at PT 6. (B) The corresponding rate constant for glucose reduction (KITT), calculated over the initial slope of the ITT glucose response curve from 0 to 30 min, was significantly reduced for GW2 vs CON/S. (C) Blood glucose values, expressed as a percent of baseline are plotted versus time. (D) The corresponding inverse integrated area (AUC) of the percent basal glucose curve (Inverse AUCITTglucose) shows a significant decrease for GW2 vs CON/S. (E–H) Absolute blood glucose concentrations measured during IP ITT at PT 192 show no group differences in glycemia response over 120 min post-injection (E), KITT (F), glycemia expressed as percent baseline (G) and corresponding inverse AUCITTglucose (H). Body weight (I, K): Body weight, monitored before (PRE), during (TRT) and post (PT) GW exposure, shows no difference vs CON/S. QMR (J, L): Measurements indicate that GW exposure did not affect fat and lean mass composition measured at PT 25 (J) and PT 139–166 (L). Stress alone did not influence any parameters. Values are expressed as mean ± s.e.m. *Significantly different from CON/S, *p < .05. Ŝignificantly different from GW1, ^p < .05, ^^p < .01. A–D, I, J: n = 6–9/group and E-H, K, L: n = 5–10/group. PRE, pretreatment; PT, post-treatment; QMR, quantitative magnetic resonance; TRT, treatment. GW1, 6.5 mg/kg b.i.d.; GW2, 8.7 mg/kg.
Body weight was monitored during select time points during treatment. No effects of GW agent exposure were observed between GW and control groups (Fig. 2I, K). Body composition was also assessed by QMR. Fig. 2J and L shows that there were no differences in lean mass nor fat mass. Taken together, these results suggest that GW agents at the GW2 dose produce a transient decrease in insulin sensitivity and possibly glucose utilization/clearance that is not due to the effect of stress nor is driven by changes in body mass composition.
3.2. GW2 produces persistent exercise intolerance without deficits in locomotion or sensorimotor integration
3.2.1. Exercise endurance
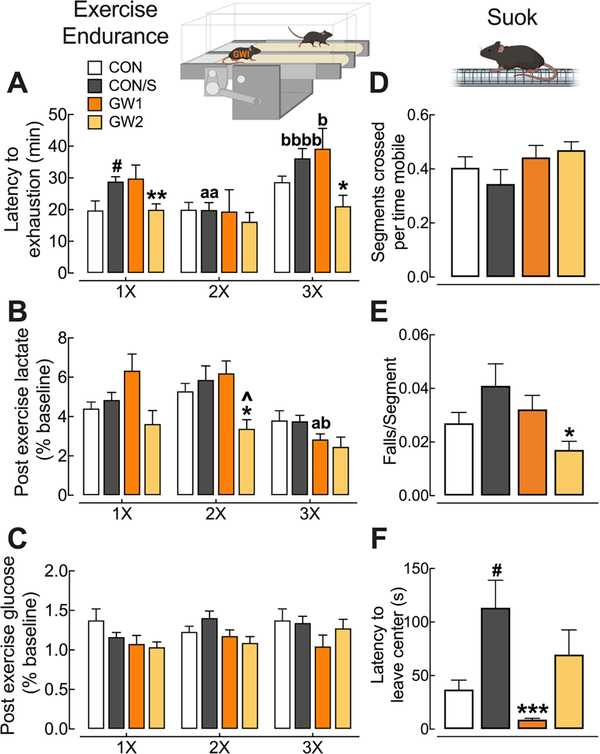
Latency to fatigue was assessed using an Exercise Endurance (EE) protocol at PT 23 (1×), PT 109 (2×) and PT 161 (3×). Between-group comparisons indicated that only GW2 showed reduced latency to exhaustion relative to CON/S at PT 23 (p < .01) (Fig. 3A). This deficit persisted at PT 161 (p < .05). This is not likely due to an effect of stress since CON/S displayed better endurance relative to CON at 1× (p < .05). In contrast, GW1 did not show exercise intolerance vs CON/S and, surprisingly, improved latency at PT 161 (3×) relative to 2nd testing (2×) (p < .05). The opposite profiles of GW1 and GW2 relative to CON/S indicate that the PB dose and not stress may explain exaggerated exercise fatigue seen in GW2 at early testing. Blood lactate was measured before and immediately after mice underwent EE in order to calculate post-exercise lactate values as a percent of baseline. Mean values were significantly reduced at PT 109 in GW2 relative to CON/S and GW1 (p < .05) (Fig. 3B). Interestingly, mean values for GW1 at PT 161 were significantly different from both its earlier time points, indicating a reduction in the magnitude increase of lactate across time (p < .05). For blood glucose no differences were found across groups or across time (Fig. 3C).
Fig. 3.
GW2 produces persistent exercise intolerance without deficits in locomotion or sensorimotor processing. Mice were subjected to repeat tests of exercise endurance at PT 23 (1×), PT 109 (2×), and PT 161 (3×) and SUOK test at PT 10. Exercise endurance (A–C): (A) Mean scores on latency to exhaustion were significantly reduced for GW2 at 1× and 3× vs CON/S. (B,C) Corresponding mean post-exercise blood lactate (B) were not elevated in any group and post-exercise glucose levels (C) were not significantly affected by exercise. SUOK test (D–F): (D) Locomotor activity, expressed as segments crossed over time spent mobile at PT 10 showed no intergroup differences. (E) Corresponding falls per segment crossed and (F) latency to leave center indicate no sensorimotor deficits for GW groups. Values are expressed as mean ± s.e.m. aSignificantly different from x1, ap < .05, aap < 0.01. bSignificantly different from x2, bp < 0.05, bbbbp < 0.0001. *Significantly different from CON/S at corresponding time point, *p < .05, **p < .01, ***p < .001. Ŝignificantly different from GW1 at corresponding time point, ^p < .05. #Significantly different from CON at corresponding time point, #p < .05, n = 5–10/group (A-C) and n = 12–15/group (D-F). GW1, 6.5 mg/kg b.i.d.; GW2, 8.7 mg/kg.
3.2.2. SUOK
Sensorimotor ability measured on the SUOK test showed no differences across groups for locomotor activity (Fig. 3D). The test, however, indicated a significant reduction in the falls per segments crossed in GW2 vs CON/S (p < .05) (Fig. 3E). Latency to leave center, a measure of anxiety, was significantly lower in GW1 relative to CON/S (p < .05) (Fig. 3F). There were no group differences in additional locomotor parameters (Supplementary information, Supplementary Fig. 1).
3.3. GW agent exposure produced no adverse effects on motor coordination
For evaluation of motor coordination we used the Rotarod test (Supplementary Information, Supplementary Fig. 2) and within-group comparisons showed that the CON/S, GW1 and GW2 groups performed better in the fourth trial, compared to the first trial (p < .05), indicating improved motor learning for each group. Differences across groups included increased latency to fall in GW1 vs CON/S (p < .05) and reduced latency in CON/S vs CON in the second trial (p < .01).
3.4. GW agent exposure compromises fear-associated learning without affecting pain sensitivity
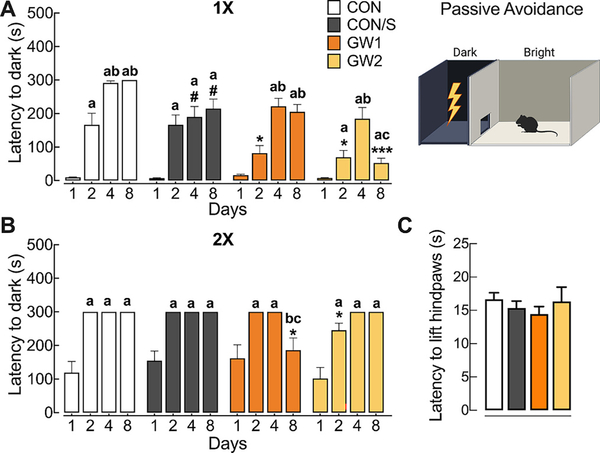
The passive avoidance learning and memory test (PAT) with foot-shock was used to assess associative learning and memory. At PT 34–70, mice were subjected to an acquisition trial on day 1 and tested for latency to enter the dark chamber on retention trials performed at days 2, 3 and 7. Within group comparisons showed significantly increased time to enter the dark chamber on day 2, indicating memory retention after a 24 h period for CON, CON/S and GW2 but not GW1. Across-group comparisons showed impaired retention in GW1 and GW2 since latency to enter dark was significantly shorter on day 2 relative to CON/S (Fig. 4A; p < .05). All groups showed memory retention that persisted out to 7 days, except GW2 representing a long-term deficit in memory retention (p < .05, vs days 2 and 3 and to CON/S, Fig. 4A; p < .001). Mice were subjected to a second PAT at PT 110–120 (Fig. 4B). All groups showed a high baseline for acquisition suggesting mice had remembered to associate the context with the unconditioned stimulus. Nevertheless, GW1 and GW2 showed memory deficits, i.e., shorter latency to enter dark chamber on day 2 (GW2) relative to CON/S (p < .05). In contrast, GW1 showed less retention at day 7 relative to CON/S (p < .05) and vs day 2 and 3 (p < .05).
Fig. 4.
GW agent exposure compromises fear-associated learning without affecting pain sensitivity. Passive avoidance test (PAT, A, B): Mice were subjected to two passive avoidance tests (1× and 2×) at time points indicated. PAT with foot shock was conducted to assess fear-associated learning as measured by hesitation to leave bright chamber on days 2–8 after training (day 1). (A) PAT at PT 34–70 (1×) showed that CON and CON/S avoided aversive dark chamber on days 2–8. In contrast, GW1 and GW2 took two days longer to accomplish memory retention and GW2 group also presented a deficit at day 8 when compared to CON/S. (B) PAT at PT 110–120 (2×). Increased latency scores over successive test trials were observed for all groups with 2 exceptions. Intergroup differences indicated reduced memory retention for GW1 at day 8 and GW2 at day 2 relative to CON/S. Hot plate test at PT 191 (C) Mean scores on withdrawal latency show no group differences. Values are expressed as mean ± s.e.m. #significantly different from CON at corresponding day (p < .05). *Significantly different from CON/S at corresponding day, *p < .05, ***p < .001. aSignificantly different from day 1, ap < .05; bSignificantly different from day 2, bp < .05; cSignificantly different from day 3, cp < .05; n = 13–17/group (A,B), n = 5–10/group (C). GW1, 6.5 mg/kg b.i.d.; GW2, 8.7 mg/kg.
A hot plate test was used to assess nociceptive threshold to test the possibility that GW groups performed worse on passive avoidance test due to elevated pain threshold. No statistical significance among groups was found at PT 190, suggesting that reduced associative learning and memory was not confounded by hyper or hypoalgesia (Fig. 4C).
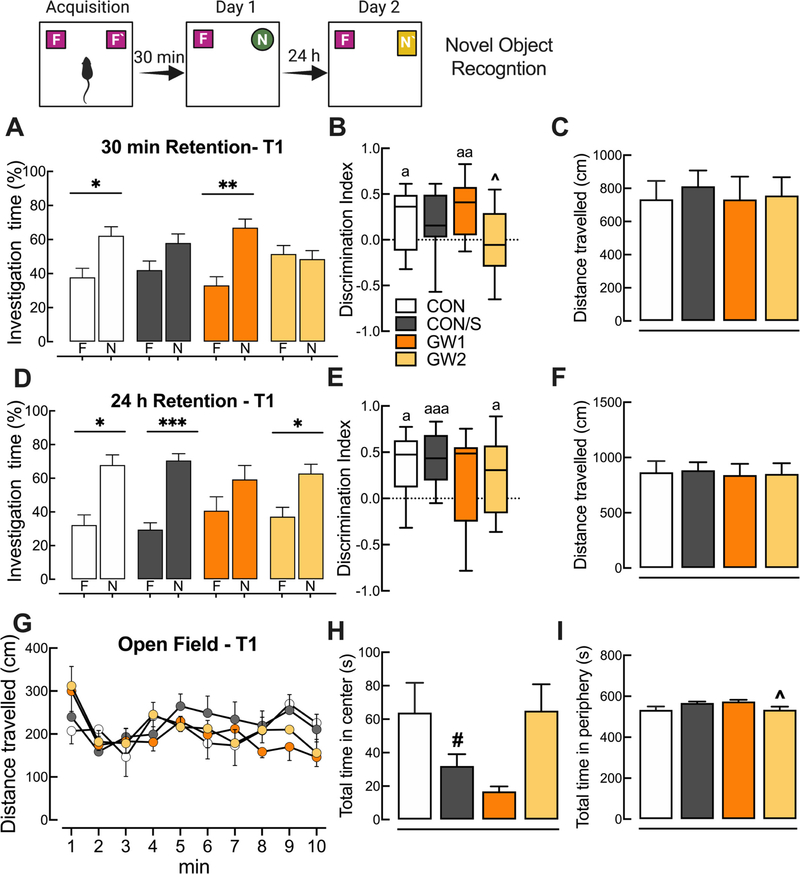
3.5. GW agent exposure produces short- and long-term object recognition memory deficits without anxiety or locomotion alterations
3.5.1. Novel object recognition
Results obtained on the novel object recognition test show that at 30 min retention time the GW2 and CON/S groups did not recognize the novel over familiar objects (Fig. 5A), suggesting that altered short-term memory is possibly due to stress. In Fig. 5B, the corresponding discrimination index values were significantly different from 0, indicating a significant preference in exploring the novel object and normal recognition memory for all groups except CON/S and GW2 (p < .05–.01). Mean NORT scores for 24 h retention indicate that all groups except the GW1 group showed a significant preference for the novel object (Fig. 5D; p < .05 and p < .001) and a discrimination index that was significantly greater than 0 (Fig. 5E; p < .05 and p < .001). Therefore, GW1 group displayed deficient long-term recognition memory. It should be noted that performance on the 24 h retention test cannot be completely separated from the carryover effects of the 30 min test since the same mice were tested for recognition memory after both retention periods. These changes in investigation of novel vs familiar objects were not due to reduced locomotion, measured as distance travelled in the open field arena at Phase I (Fig. 5C) and Phase II (Fig. 5F).
Fig. 5.
GW agent exposure produces deficits in short- and long-term novel object recognition memory without anxiety or locomotion alterations. Novel object recognition test, A–F: Short- and long-term memories were tested in Phase I (30 min) and Phase II (24 h retention) performed at PT 17 (T1). (A) After a 30 min retention period GW2 and CON/S spent equal time investigating familiar and novel objects, indicating a short-term memory deficit that is possibly due to an effect of stress alone. (B) Mean discrimination index scores significantly greater than 0 indicate that all mice except CON/S and GW2 preferred the novel vs familiar object. (D) After 24 h retention GW1 behaved abnormally and showed no preference for a new novel object vs familiar relative to CON/S. (E) Mean corresponding discrimination index indicates that all groups except GW1 showed an overall preference for the novel object but no across-group differences were seen. Changes in investigation were not due to immobility in the open field arena prior to Phase I (C) and Phase II (F). Open field test (G–I): (G) There were no group differences on total distance travelled in the open field arena. (H–I) Average total time spent in center (H) and periphery zone (I) indicated no reduction in time spent in center for GW groups vs CON/S. Values are expressed as mean ± s.e.m. except for those in B, E which are expressed as median and inter quartile range with whiskers representing minimum and maximum values. *Significantly different from time exploring familiar object; *p < .05, **p < .01, ***p < .001. Ŝignificantly different from GW1, ^p < .05; aSignificantly different from a hypothetical discrimination index of 0, ap < .05, aap < .01aaap < .001. #Significantly different from CON. F, familiar. N, novel. A–F, n = 10–13/group, G-I, n = 6–9/group. GW1, 6.5 mg/kg b.i.d.; GW2, 8.7 mg/kg.
3.5.2. Open field tests (OFT)
Prior to the acquisition phase of NORT, mouse locomotion and anxiety were evaluated while mice explored an open field arena for 10 min at PT 17. There were no group differences in total distance travelled (Fig. 5G). Similarly, there was no difference observed between either of the GW groups vs CON/S in the total time spent in the periphery over the center (Fig. 5H, I), suggesting that investigation time in NORT was not influenced by increased anxiety. The GW2 group remained less time in the periphery when compared to GW1 (p < .05; Fig. 5I).
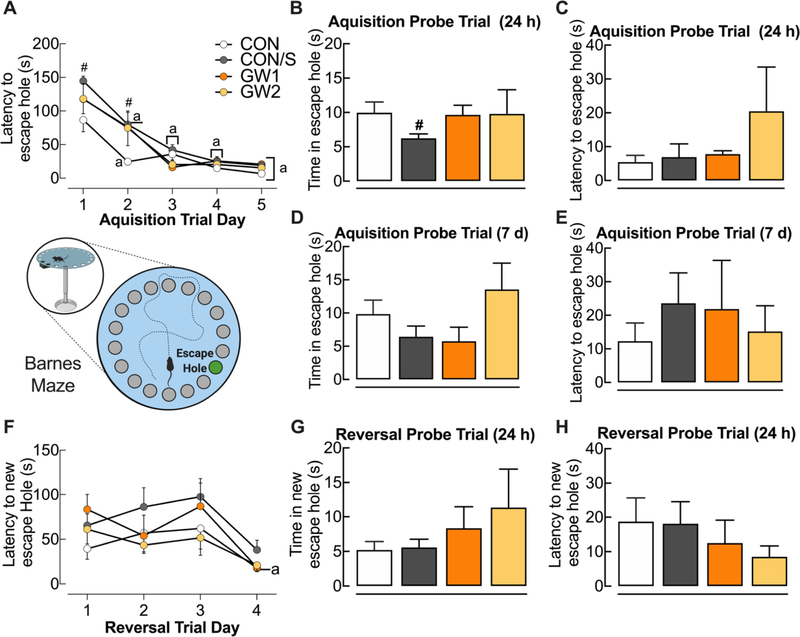
3.6. GW agents do not affect spatial learning or memory retrieval
We used an extended Barnes maze (BM) protocol to assess spatial learning (acquisition trials) and memory retrieval (acquisition/reversal probe trials) and cognitive flexibility (reversal learning). Within group comparisons of acquisition trials, which were conducted at PT 172, showed that CON and CON/S groups had learned to locate the escape hole by day 2 of the 5-day period. In contrast, both GW groups learned only by day 3 (Fig. 6A). Across group comparisons revealed that CON/S showed increased latency to escape on Day 1 and 2 relative to CON (Fig. 6A; p < .05). During the single probe acquisition conducted 24 h later to assess short-term spatial memory, no group differences were seen except for the memory-reducing effect of stress (Fig. 6B; p < .05). There were no abnormalities in the latency to the escape hole for GW1 and GW2 relative to CON/S (Fig. 6C). During the acquisition probe trial conducted 7 days later to measure long-term spatial memory, there were no differences in time spent in the escape hole nor latency to the escape hole (Fig. 6D, E). We also implemented reversal learning trials in order to assess the cognitive flexibility in relearning a new location. On the reversal learning acquisition trials, there were no differences seen between CON/S and GW1 or GW2 in the ability to learn the new position of the escape hole (Fig. 6F). This finding was also confirmed in the single short-term reversal probe trial conducted 24 h later as assessed by the time spent in and latency to the new escape hole (Fig. 6G, H).
Fig. 6.
GW treatment does not affect spatial learning and memory retrieval. Acquisition learning trials were conducted on the Barnes maze at PT 172. (A) Intergroup comparisons indicated increased latency to escape hole for CON/S vs CON at Days 1, 2. Unlike CON and CON/S, which learned the location of escape hole by Day 2, GW groups showed delayed learning until Day 3. (B) All mice behaved similarly with regard to time in the escape hole, except CON/S who spent less time in escape. (C) Latency to reach the escape hole was assessed in a single probe trial conducted 24 h later. (D-E) There were no differences in time in (D) and latency to reach (E) escape hole in a single probe trial conducted 7 days later. (F–H) During reversal learning trials, only the GW1 group learned to find the new escape location by Trial 4. There were no group differences for time in new escape location (G) nor latency to reach new escape hole (H). Values are expressed as mean ± s.e.m. #Significantly different from CON, #p < .05. aSignificantly different from Trial 1 for corresponding group, ap < .05, n = 5–10/group. GW1, 6.5 mg/kg b.i.d.; GW2, 8.7 mg/kg.
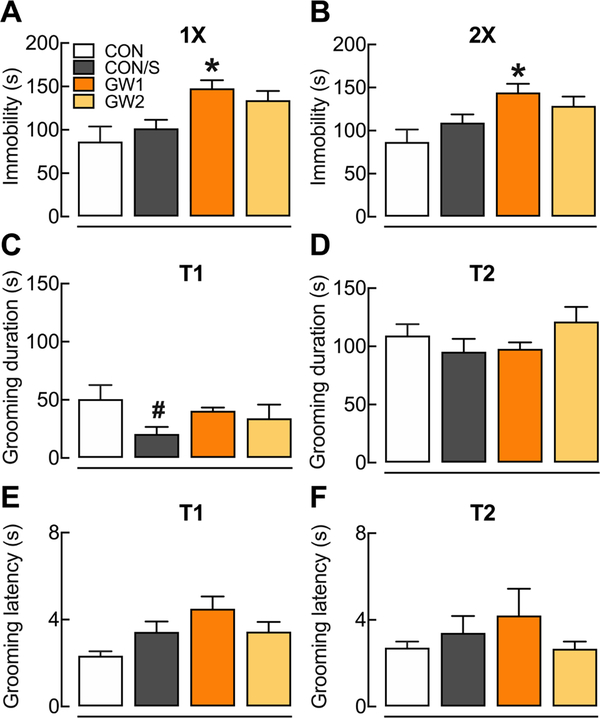
3.7. GW1 shows despair-like behavior
Depressive-like behaviors were assessed with repeated testing on the FST at PT 55 (1×) and PT 84 (2×) and on the SST at different time points at PT 28 (T1) and PT 140 (T2). Immobility time in FST was significantly greater indicating despair-like behavior in the GW1 group at 1× and 2×, when compared to CON/S (p < .05) (Fig. 7A, B). Regarding the self-care behavior assessed in the SST, less grooming time was observed in the CON/S group, compared to CON (Fig. 7C, p < .05) at PT 28 and no difference was found at PT 140 (Fig. 7D). Additionally, no difference was found in the latency to the first grooming at any time point (Fig. 7E, F). For depressive-like behavior assessed through TST, no difference was found across groups (Supplementary information, Supplementary Fig. 3).
Fig. 7.
GW1 shows despair-like behavior. Mice were subjected to a sucrose splash test (SST) at one of two times (T1, T2) and to the forced swim test (FST) at two successive times (1×, 2×) throughout the post-treatment period. FST (A–B): (A) FST at 1× (PT 55). (B) FST at 2× (PT 84). SST: (C) Grooming time in SST at PT 28 (T1). (E) Latency to start grooming in SST at PT 28 (T1). (D) Grooming duration in SST at PT 140 (T2) (F) Latency to start grooming in SST at PT 140. Values are expressed as mean ± s.e.m. *Compared to CON/S, *p < .05; #compared to CON, #p < .05. n = 5–10/group. GW1, 6.5 mg/kg b.i.d.; GW2, 8.7 mg/kg.
3.8. GW agents do not affect anxiety
The elevated plus maze was used to test anxiety. Supplementary information, Supplementary Fig. 4 at PT 14 shows that all mice spent significantly more time in closed vs open arms, indicating internal validity of the maze (p < .0001). However, no across group differences in time spent in the open arms were observed suggesting that GW agents do not exaggerate anxious behavior. To test for latently emerging effects we subjected mice to EPM at PT 92 and PT 145 and found similar results (Supplementary information, Supplementary Fig. 4). No differences in the total frequency of entries were seen across treated groups supporting the lack of an anxiogenic effect by GW agents (Supplementary Information, Supplementary Fig. 4).
4. Discussion
The pathophysiological processes underlying the chronic symptoms seen in GWI have not been established, curtailing effective diagnosis and treatment for this debilitating chronic disorder. One of the hurdles has been the lack of a tractable animal model that recapitulates the most prevalent symptoms in GWI, such as debilitating fatigue and cognitive decline [62]. Here, we characterized a GWI mouse model with exaggerated exercise intolerance and impaired associative learning and object recognition memory. Notably, the GWI phenotype is driven by GW chemicals since the abnormalities in the overwhelming majority of parameters studied could not be recapitulated by stress exposure alone. Specifically, the 6.5 mg/kg, b.i.d. (GW1) did not appear to be as impactful as the single 8.7 mg/kg treatment per day received by GW2. Because GW1 mice were exposed to GW chemicals throughout the day rather than just acutely as in GW2, GW1 mice may have developed physiological adaptation (tolerance) to ACh producing changes in cholinergic receptor responses. In support of this, Dabisch and others [63] have demonstrated that overstimulation with PB, and the resultant elevation of ACh, causes increased tolerance to ACh on the ocular miotic response, pointing to oversaturation of muscarinic receptors as the likely cause. Along with this, the systemic half-life of PB in rats is roughly 30 min [64], which would imply that the muscarinic receptors of GW1 mice were more heavily saturated with PB than GW2.
We aimed to characterize the glucose metabolic health in our model since epidemiological evidence in GWVs supports an association for GWI with metabolic syndrome and obesity [65] and animal models indicate obesity worsens GWI pathology across multiple domains [66,67]. The ITT results in this study show that GW2 but not GW1 manifested insulin insensitivity (lower KITT) relative to CON/S. The former also showed recalcitrance to return to normal glycemia after insulin challenge resulting in a reduced inverse AUCITTglucose possibly representing less glucose utilization or elimination, effects that could not be attributed to obesity (increased body weight) nor reduced lean mass. These metabolic effects did not persist beyond PT 6 in contradistinction to changes in cognitive, and exercise endurance parameters. Albeit the glucose dyshomeostasis was transient, these results warn about the diabetogenic risk of GW agent exposure. In a study of Australian GWVs, multi-symptom illness was associated with elevated plasma glucose [68]. Currently, diabetes is more prevalent in GWI [22], and the additional burden of obesity and chronic intake of Western diet in the current situation of GWVs [66,67] may result in more substantive risk for accelerated brain aging, risk of dementia and cognitive impairment and fatigue symptoms [20,21].
Little is known about how GW agents may directly interfere with insulin signaling and related glucose transporters at target tissues. PB is an AChEI that can increase the level of the neurotransmitter acetylcholine (ACh), which elevates glucose utilization via GLUT4 transporters, in part, by improving vagal nerve activity [69]. Consistent with this, in obese human patients, PB, through cholinergic enhancement, increases the insulin response to glucose [70]. Complementary support from animal studies shows that the cholinergic antagonist atropine decreases the glucose disposal response to exogenously administered insulin [71,72]. Prolonged elevation of ACh levels, however, resulting from chronic AChEI application may desensitize muscarinic ACh receptors and cause subsequent blockade of ACh responses [73]. Therefore, chronic PB-mediated block of AChEI may promote cholinolytic actions on insulin signaling seen in GW2. Additionally, there is evidence that exposure to pyrethroids insecticides such as PER, is linked to increased weight gain, glucose dyshomeostasis in rodents [74] and Type II diabetes [75,76] in humans [77,78]. Altered glucose/lipid metabolism in vitro [79] and insulin resistance in mice have also been reported [80]. In humans, chronic exposure to PER has been associated with increased blood glucose levels in animals and humans and increased body mass index, a comorbidity of diabetes, in pesticide factory workers [81], although the biochemical mechanisms are poorly understood. The most prevalent complaint in GWI patients is severe and debilitating fatigue [17]. Of particular interest is our finding that GW2 displayed marked exercise intolerance up to PT 161 relative to CON/S, offering translational value since chronic fatigue continues to be a predominant symptom reported by ill GWVs [82]. While there is sufficient evidence from self reports that GWVs suffer from chronic fatigue, a more detailed analysis is required to understand the effects of exercise or physical exertion on exacerbating GW phenotypes [18,83–85]. In our study, exercise intolerance in GW2 cannot be ascribed to elevated lactate levels that may result in muscular fatigue [86], since post-exercise lactatemia was normal relative to control. Similarly, there were no group differences in post-exercise changes in glycemia that could explain exercise intolerance, indicating other physiological processes may be responsible. For example, reduced muscle and/or subcortical levels of ATP have been found in GWI mice [87]. Alternatively, morphological data from PB-treated mice showing structural changes in skeletal muscle [88] may explain, in part, intolerance to exercise in GW2. Central mechanisms may also contribute to exercise fatigue such as increased tryptophan-kynurenic acid pathway activity within the hypothalamus-hippocampal circuit and disturbances in the serotonergic system implicated in different fatigue models [89,90]. The exercise-intolerant phenotype seen in GW2 was coincident with reduced associative learning on PAT that can be studied using the same mouse model. It is important to note that ill GWVs have been suffering from persistent cognitive and fatigue deficits for 30 years. Veterans with GWI have higher rates of adverse health conditions and present poorer physical functioning compared to GW-era non-deployed veterans likely due to chronic fatigue symptoms that promote a sedentary lifestyle [20]. GWI veterans seem to be showing accelerated aging and presenting higher rates and earlier onset of medical conditions, such as diabetes [20].
Along with chronic fatigue, impaired cognition is one of the most debilitating symptoms of GWI [91]. Symptomatic GWVs present deficits in visuospatial abilities, executive functioning, and learning and memory [92] which severely affect quality of life. Using a passive avoidance test that measures associative learning and memory, we showed that, when tested at PT 34–70, GW1 and GW2 took 2 days longer to learn the aversive nature of the dark chamber, relative to CON/S. GW2 also showed memory loss at day 8, suggesting a severe learning impairment. On repeat testing, occurring at an extended time point, i.e., PT 110–120, GW1 showed memory loss only at day 8 and GW2 showed memory impairment at day 2. Therefore, the PB dose may dictate the nature of the impairment in associative learning and memory. The amygdala and hippocampus and various cortical areas are part of the neural network that subserves passive avoidance learning [93,94], but NMDA-receptors in hippocampus are especially necessary for contextual learning [93]. This coincides with hippocampal dysfunction and cognitive problems seen in GWVs [95–97]. However, our PAT findings differ from those studied in a rat model exposed to Sarin and PB alone, and in combination, for which no effect of exposure was seen [98]. It should be noted that combined PB and Sarin treatment has been reported to produce elevated nociceptive threshold at PT 16 weeks which may influence results reported previously [98]. The differences on PAT performance shown here could not be ascribed to an elevated pain threshold or exaggerated anxiogenic effects towards the aversive electric shock of PAT caused by GW agents, since no abnormal effects were observed on the hot plate test or EPM, respectively.
We also report a dose-dependent GW agent-induced reduction in long-term object recognition memory (GW1) on NORT, that requires the integrity of the hippocampus, perirhinal cortex and prefrontal cortex [99,100]. Wang and colleagues showed long-term recognition memory deficits in male but not female C57BL/6 mice chronically exposed to GW chemicals (1.3 mg/kg PB P.O., 0.13 mg/kg PER and 40 mg/kg DEET topical) [87]. Similar results have been reported in rats [101]. Reduced short-term (30 min) object recognition memory was also seen in GW2 and CON/S suggesting the outcome was likely due to stress alone. Therefore, it is important to use the proper control to parse out the unique effects of GW chemicals. Distance travelled during NORT (open field test) showed no group differences ruling out the possibility that differential NORT scores were due to deficits in general sensorimotor ability. Our experimental results align well with the impaired central executive processing seen in symptomatic GWVs displayed as distinctive prefrontal cortical activity during a working memory challenge task on fMRI, when compared to civilian controls [7,102]. Since GWVs have self-reported visual memory decrements [103] we also examined the effects of GW agents on spatial memory using the Barnes maze test [103]. Our results indicate only minor changes, namely that both GW groups require a longer acquisition period to learn the location of escape hole compared to CON/S at 6 months post exposure. Previous GW rodent models have found more profound deficits when tested similarly at 5–22.5 month post exposure [87,104–107]. In combination, the results of the cognitive tests performed indicate several cognitive effects of GW agents that likely vary by dose of chemicals and time following exposure.
Since a combination of GW agents was used to mimic GW exposure, it has been difficult to determine which chemicals are the most salient in producing GW phenotypes. However, we found differential phenotypes using two different PB doses suggesting pharmacological actions of PB and/or interactions with PER and/or DEET are driving the phenotypic changes. Several epidemiological studies have reported a link between exposure to a AChEI, such as pyridostigmine bromide (PB), and chronic symptoms in GW veterans [8]. Indeed, even though PB does not normally cross the blood-brain barrier, stress has been shown to enhance PB entry and affect acetylcholinesterase (AChE) activity in rats [13]. These changes may interfere with basal forebrain cholinergic circuits participating in domains altered in GWI such as memory, attention and sleep [11,15]. Within the cortex, structural changes such as reduced neurogenesis, neurodegeneration and inflammation in the hippocampus may underlie object memory dysfunction (NORT) and associative learning deficits (PAT) [108] and have been previously shown to occur after GW agent exposure [11,91]. Therefore, as a follow up to this study demonstrating differential phenotypes for GW2 and GW1, we compared neuroinflammatory transcriptional signatures, and brain IL-6 levels as well as plasma endotoxin and the gut microbiome community structure to find molecular/pathological correlates that may specifically drive the GW2 cognitive and fatigue phenotype [1].
Neurological and psychiatric symptoms including cognitive impairment, attention deficits, depression and anxiety are widely reported among GWI patients [109]. This is hypothesized to be due to stress-induced disruption of the blood-brain barrier allowing PB penetration into the brain [13] and disrupting the homeostatic balance in synaptic acetylcholine signaling that is required for mood regulations and stress reactivity [110]. Consistent with other studies, here we show that GW1 (but not GW2) displayed a significant increase in time spent immobile on FST, corroborating previous findings of depressive-like effect of GW agent exposure in animal models [11,87,104]. The immobility observed in GW1 is not likely confounded by fatigue since GW1 reached exercise exhaustion at a normative time (>25 min) and no locomotor deficits were seen in the open field test. On a separate test, the SST, that measures self-care, we observed only the effect of stress, but not of GW agents. The differential effects of FST and SST could be due to the different types of depressive-like behavior that each test measures. While the FST measures behavioral despair, that parallel with symptoms of despair and hopelessness seen in depression [57,111], the SST assesses the grooming behavior, considered a form of motivational behavior that parallels other symptoms of depression, such as the apathetic behavior [112]. These findings corroborate the increased rates of mood disorders, especially depression, reported by GWVs [113].
With regard to anxiety, previous reports showed increased anxiety following administration of GW agents and stress at 3 months post treatment compared to vehicle control [23,106]. While our results obtained on EPM do not support an anxiogenic effect of GW agent treatment, CON/S did increase latency to leave center on the SUOK test and produced less time in center of OFT. Therefore, the discrepancy may be due to the type of control group selected for comparison. Our findings on cognitive and mood domains are consistent with previous published results showing that chronic GW1 agent exposure promoted depressive-like behavior but not enhanced anxiety, a combination seem in some rat [25] and mouse models [106] but not others showing enhanced anxiety as well [11,23,87,114]. Importantly, the cognitive and exercise intolerance displayed prominently by GW2 did not appear to parallel a depressive-like nor increased anxiety phenotype, indicating different circuits are involved in the pathological manifestations of the GW2 PB dose.
Supplementary Material
Acknowledgements
We acknowledge technical assistance from V. Wagner, NRSC Graduate Program, UCR. We thank Drs. K. Huffman, W. Saltzman for access to behavioral apparati and Drs. I. Ethell, F. Sladek, S. Tiwari-Woodruff for breeder mice. We thank Dr. T. Garland for use of EchoMRI (QMR) machine and help with exercise endurance protocol. We thank the Santhakumar and Anderson labs for training and use of the Barnes maze. We are grateful to Drs. B. Wong (Noldus) and Hartman for training and access to Ethovision. Images were created using BioRender.com.
Funding
GWIRP Department of Defense W81XWH-19-1-0802 (to M.C.C. and N.I.z.N.), UC Riverside GRMP (E.V.K.), UC Riverside Mini Grant (A.E.B.; J.T.), Sigma Xi GIAR (A.E.B.); American Physiological Society STRIDE Fellowship (to A.E.B.), National Institute of General Medical Sciences R35GM124724 and National Institutes of Health NIAID grants R01AI157106 (to A.H.).
Abbreviations:
- AChEI
acetylcholinesterase inhibitor
- AUC
area under curve
- b.i.d.
bis in die, twice daily
- CNS
central nervous system
- CF
chronic fatigue
- DEET
N,N-diethyl-meta-toluamide
- DG
dentate gyrus
- DMSO
dimethyl sulfoxide
- EE
exercise endurance
- EtOH
ethanol
- FITC
fluorescein isothiocyanate
- FST
forced swim test
- GW
Gulf War
- GWI
Gulf War Illness
- (GWVs)
Gulf War veterans
- HPT
hot plate test
- IL-1β
interleukin-1β
- ip
intraperitoneal
- ITT
insulin tolerance test
- KITT
glucose reduction rate
- MOL
molecular layer
- MRI
magnetic resonance imaging
- NORT
novel object recognition test
- OFT
open field test
- PAT
passive avoidance task
- PB
pyridostigmine bromide
- PER
permethrin
- P.O.
per os, by mouth
- PT
post-treatment
- q.d.
quaque die, once daily
- QMR
quantitative magnetic resonance
- SST
sucrose splash test
- T
time point
- TRT
treatment
- TST
tail suspension test
- UCS
unconditioned stimulus
Footnotes
Ethics approval and consent to participate
Care and treatment of animals was performed in accordance with guidelines from and approved by the University of California, Riverside Institutional Animal Care and Use Committee (AUP# 20210024, 20200020 and 20180067).
Declaration of competing interest
No competing interests to declare.
Consent for publication
Not applicable.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.lfs.2021.120094.
References
- [1].Kozlova EV, Carabelli B, Bishay AE, Liu R, Denys ME, Macbeth JC, Piamthai V, Crawford MS, McCole DF, zur Nieden NI, Hsiao A, Curras-Collazo MC, Induction of distinct neuroinflammatory markers and gut dysbiosis by differential pyridostigmine bromide dosing in a chronic mouse model of GWI showing persistent exercise fatigue and cognitive impairment, Life Sci. (2022), 120153, 10.1016/j.lfs.2021.120153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Porter B, Long K, Rull RP, Dursa EK, Millennium Cohort Study Team, Prevalence of chronic multisymptom illness/gulf war illness over time among millennium cohort participants, 2001 to 2016, J. Occup. Environ. Med 62 (2020) 4–10, 10.1097/JOM.0000000000001716. [DOI] [PubMed] [Google Scholar]
- [3].Steele L, Prevalence and patterns of gulf war illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service, Am. J. Epidemiol. 152 (2000) 992–1002, 10.1093/aje/152.10.992. [DOI] [PubMed] [Google Scholar]
- [4].Institute of Medicine, Board on the Health of Select Populations, Committee on Gulf War and Health, Health Effects of Serving in the Gulf War, Update 2009, Gulf War and Health: Volume 8: Update of Health Effects of Serving in the Gulf War, National Academies Press, 2010. https://play.google.com/store/books/details?id=dzJkAgAAQBAJ. [Google Scholar]
- [5].Mawson AR, Croft AM, Gulf War illness: unifying hypothesis for a continuing health problem, Int. J. Environ. Res. Public Health 16 (2019), 10.3390/ijerph16010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Engdahl BE, James LM, Miller RD, Leuthold AC, Lewis SM, Carpenter AF, Georgopoulos AP, Brain function in gulf war illness (GWI) and associated mental health comorbidities 3 (2018) 24–34. https://www.ncbi.nlm.nih.gov/pubmed/30882065. [PMC free article] [PubMed] [Google Scholar]
- [7].White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R, Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment, Cortex 74 (2016) 449–475, 10.1016/j.cortex.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Golomb BA, Acetylcholinesterase inhibitors and Gulf War illnesses, Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 4295–4300, 10.1073/pnas.0711986105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abdullah L, Evans JE, Bishop A, Reed JM, Crynen G, Phillips J, Pelot R, Mullan MA, Ferro A, Mullan CM, Mullan MJ, Ait-Ghezala G, Crawford FC, Lipidomic profiling of phosphocholine-containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents, Neuromolecular Med. 14 (2012) 349–361, 10.1007/s12017-012-8192-z. [DOI] [PubMed] [Google Scholar]
- [10].Abdel-Rahman A, Shetty AK, Abou-Donia MB, Disruption of the blood-brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome, Neurobiol. Dis. 10 (2002) 306–326, 10.1006/nbdi.2002.0524. [DOI] [PubMed] [Google Scholar]
- [11].Parihar VK, Hattiangady B, Shuai B, Shetty AK, Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus, Neuropsychopharmacology 38 (2013) 2348–2362, 10.1038/npp.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abou-Donia MB, Conboy LA, Kokkotou E, Jacobson E, Elmasry EM, Elkafrawy P, Neely M, dale’Bass CR, Sullivan K, Screening for novel central nervous system biomarkers in veterans with Gulf War Illness, Neurotoxicol. Teratol 61 (2017) 36–46, 10.1016/j.ntt.2017.03.002. [DOI] [PubMed] [Google Scholar]
- [13].Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I, Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response, Nat. Med. 2 (1996) 1382–1385, 10.1038/nm1296-1382. [DOI] [PubMed] [Google Scholar]
- [14].Parikh V, Kozak R, Martinez V, Sarter M, Prefrontal acetylcholine release controls cue detection on multiple timescales, Neuron 56 (2007) 141–154, 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hernandez S, Fried DE, Grubišić V, McClain JL, Gulbransen BD, Gastrointestinal neuroimmune disruption in a mouse model of Gulf War illness, FASEB J. 33 (2019) 6168–6184, 10.1096/fj.201802572R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Engineering National Academies of Sciences and Medicine, Institute of Medicine, Board on the Health of Select Populations, Committee on Gulf War and Health, Volume 10: Update of Health Effects of Serving in the Gulf War, Gulf War and Health: Volume 10: Update of Health Effects of Serving in the Gulf War, 2016, National Academies Press, 2016, 10.17226/21840. [DOI] [PubMed] [Google Scholar]
- [17].Institute of Medicine, Board on the Health of Select Populations, Committee on the Development of a Consensus Case Definition for Chronic Multisymptom Illness in 1990–1991 Gulf War Veterans, Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined, National Academies Press, 2014, 10.17226/18623. [DOI] [PubMed] [Google Scholar]
- [18].McCauley LA, Joos SK, Barkhuizen A, Shuell T, Tyree WA, Bourdette DN, Chronic fatigue in a population-based study of Gulf War veterans, Arch. Environ. Health 57 (2002) 340–348, 10.1080/00039890209601419. [DOI] [PubMed] [Google Scholar]
- [19].Kang HK, Li B, Mahan CM, Eisen SA, Engel CC, Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years, J. Occup. Environ. Med. 51 (2009) 401–410, 10.1097/JOM.0b013e3181a2feeb. [DOI] [PubMed] [Google Scholar]
- [20].Zundel CG, Heeren T, Grasso CM, Spiro A 3rd, Proctor SP, Sullivan K, Krengel M, Changes in health status in the Ft. Devens Gulf War Veterans Cohort: 1997–2017, Neurosci. Insights 15 (2020), 2633105520952675, 10.1177/2633105520952675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goedendorp MM, Tack CJ, Steggink E, Bloot L, Bazelmans E, Knoop H, Chronic fatigue in type 1 diabetes: highly prevalent but not explained by hyperglycemia or glucose variability, Diabetes Care 37 (2014) 73–80, 10.2337/dc13-0515. [DOI] [PubMed] [Google Scholar]
- [22].Zundel CG, Krengel MH, Heeren T, Yee MK, Grasso CM, Janulewicz Lloyd PA, Coughlin SS, Sullivan K, Rates of chronic medical conditions in 1991 Gulf War veterans compared to the general population, Int. J. Environ. Res. Public Health 16 (2019), 10.3390/ijerph16060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carreras I, Aytan N, Mellott T, Choi J-K, Lehar M, Crabtree L, Leite-Morris K, Jenkins BG, Blusztajn JK, Dedeoglu A, Anxiety, neuroinflammation, cholinergic and GABAergic abnormalities are early markers of Gulf War illness in a mouse model of the disease, Brain Res. 1681 (2018) 34–43, 10.1016/j.brainres.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].C. RA on Gulf War Veterans’ Illnesses, Gulf War Illness and the Health of Gulf War Veterans: Research Update and Recommendations, 2009–2013, US Government Printing Office, 2014. [Google Scholar]
- [25].Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK, Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory, Mol. Psychiatry 16 (2011) 171–183, 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Institute of Medicine, Division of Health Promotion and Disease Prevention, Committee on Health Effects Associated with Exposures During the Gulf War, Gulf War and Health: Volume 1: Depleted Uranium, Sarin, Pyridostigmine Bromide, and Vaccines, National Academies Press, 2000. https://play.google.com/store/books/details?id=93r7zAEACAAJ. [PubMed] [Google Scholar]
- [27].Nair AB, Jacob S, A simple practice guide for dose conversion between animals and human, J. Basic Clin. Physiol. Pharmacol. 7 (2016) 27–31, 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].U.S. Epa, ORD, Recommended use of body weight 3/4 as the default method in derivation of the oral reference dose. https://www.epa.gov/risk/recommended-use-body-weight-34-default-method-derivation-oral-reference-dose, 2013. (Accessed 31 August 2021).
- [29].Tinsley FC, Taicher GZ, Heiman ML, Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis, Obes. Res. 12 (2004) 150–160, 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- [30].Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM, Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents, Obesity 18 (2010) 1652–1659, 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Morley LA, Gomez TH, Goldman JL, Flores R, Robinson MA, Accuracy of 5 point-of-care glucometers in C57BL/6J mice, J. Am. Assoc. Lab. Anim. Sci. 57 (2018) 44–50. https://www.ncbi.nlm.nih.gov/pubmed/29402351. [PMC free article] [PubMed] [Google Scholar]
- [32].Cresto JC, Lavine RL, Buchly ML, Penhos JC, Bhathena SJ, Recant L, Half life of injected 125I-insulin in control and ob/ob mice, Acta Physiol. Lat. Am. 27 (1977) 7–15. https://www.ncbi.nlm.nih.gov/pubmed/616173. [PubMed] [Google Scholar]
- [33].Lundbaek K, Intravenous glucose tolerance as a tool in definition and diagnosis of diabetes mellitus, Br. Med. J. 1 (1962) 1507–1513, 10.1136/bmj.1.5291.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP, NIH mouse metabolic phenotyping center consortium, standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice, Dis. Model. Mech. 3 (2010) 525–534, 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Friard O, Gamba M, BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations, Methods Ecol. Evol. 7 (2016) 1325–1330, 10.1111/2041-210x.12584. [DOI] [Google Scholar]
- [36].Meek TH, Lonquich BP, Hannon RM, Garland T Jr., Endurance capacity of mice selectively bred for high voluntary wheel running, J. Exp. Biol. 212 (2009) 2908–2917, 10.1242/jeb.028886. [DOI] [PubMed] [Google Scholar]
- [37].Bouganim S, Bergdahl A, Constructing an inexpensive and versatile homemade rodent treadmill, Lab. Anim 46 (2017) 67–69, 10.1038/laban.1196. [DOI] [PubMed] [Google Scholar]
- [38].Kalueff AV, Keisala T, Minasyan A, Kumar SR, LaPorte JL, Murphy DL, Tuohimaa P, The regular and light–dark Suok tests of anxiety and sensorimotor integration: utility for behavioral characterization in laboratory rodents, Nat. Protoc. 3 (2008) 129–136, 10.1038/nprot.2007.516. [DOI] [PubMed] [Google Scholar]
- [39].Kaesermann HP, Stretched attend posture, a non-social form of ambivalence, is sensitive to a conflict-reducing drug action, Psychopharmacology 89 (1986) 31–37, 10.1007/BF00175185. [DOI] [PubMed] [Google Scholar]
- [40].Benneh CK, Biney RP, Adongo DW, Mante PK, Ampadu FA, Tandoh A, Jato J, Woode E, Depress.Res. Treat 2018 (2018) 1537371, 10.1155/2018/1537371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Abbott CW, Rohac DJ, Bottom RT, Patadia S, Huffman KJ, Prenatal ethanol exposure and neocortical development: a transgenerational model of FASD, Cereb. Cortex 28 (2018) 2908–2921, 10.1093/cercor/bhx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hikosaka O, Nakamura K, Sakai K, Nakahara H, Central mechanisms of motor skill learning, Curr. Opin. Neurobiol. 12 (2002) 217–222, 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- [43].Rustay NR, Wahlsten D, Crabbe JC, Influence of task parameters on rotarod performance and sensitivity to ethanol in mice, Behav. Brain Res. 141 (2003) 237–249, 10.1016/s0166-4328(02)00376-5. [DOI] [PubMed] [Google Scholar]
- [44].Patti CL, Zanin KA, Sanday L, Kameda SR, Fernandes-Santos L, Fernandes HA, Andersen ML, Tufik S, Frussa-Filho R, Effects of sleep deprivation on memory in mice: role of state-dependent learning, Sleep 33 (2010) 1669–1679, 10.1093/sleep/33.12.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stubley-Weatherly L, Harding JW, Wright JW, Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats, Brain Res. 716 (1996) 29–38, 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- [46].Zhang M, Moon C, Chan GC-K, Yang L, Zheng F, Conti AC, Muglia L, Muglia LJ, Storm DR, Wang H, Ca-stimulated type 8 adenylyl cyclase is required for rapid acquisition of novel spatial information and for working/episodic-like memory, J. Neurosci. 28 (2008) 4736–4744, 10.1523/JNEUROSCI.1177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Woolfe G, Macdonald AD, The evaluation of the analgesic action of pethidine hydrochloride (demerol), J. Pharmacol. Exp. Ther. 80 (1944) 300–307. https://jpet.aspetjournals.org/content/80/3/300.short. (Accessed 27 February 2021). [Google Scholar]
- [48].Minett MS, Quick K, Wood JN, Behavioral measures of pain thresholds, Curr. Protoc. Mouse Biol 1 (2011) 383–412, 10.1002/9780470942390.mo110116. [DOI] [PubMed] [Google Scholar]
- [49].Ennaceur A, Delacour J, A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data, Behav. Brain Res. 31 (1988) 47–59, 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- [50].Gillera SEA, Marinello WP, Horman BM, Phillips AL, Ruis MT, Stapleton HM, Reif DM, Patisaul HB, Sex-specific effects of perinatal FireMaster® 550 (FM 550) exposure on socioemotional behavior in prairie voles, Neurotoxicol. Teratol. 79 (2020), 106840, 10.1016/j.ntt.2019.106840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gulinello M, Mitchell HA, Chang Q, Timothy O’Brien W, Zhou Z, Abel T, Wang L, Corbin JG, Veeraragavan S, Samaco RC, Andrews NA, Fagiolini M, Cole TB, Burbacher TM, Crawley JN, Rigor and reproducibility in rodent behavioral research, Neurobiol. Learn. Mem. 165 (2019), 106780, 10.1016/j.nlm.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sánchez-Rodríguez I, Temprano-Carazo S, Nájera A, Djebari S, Yajeya J, Gruart A, Delgado-García JM, Jiménez-Díaz L, Navarro-López JD, Activation of G-protein-gated inwardly rectifying potassium (Kir3/GirK) channels rescues hippocampal functions in a mouse model of early amyloid-β pathology, Sci. Rep. 7 (2017) 14658, 10.1038/s41598-017-15306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hall CS, Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality, J. Comp. Psychol 18 (1934) 385–403, 10.1037/h0071444. [DOI] [Google Scholar]
- [54].Barnes CA, Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat, J. Comp. Physiol. Psychol. 93 (1979) 74–104, 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- [55].Gawel K, Gibula E, Marszalek-Grabska M, Filarowska J, Kotlinska JH, Assessment of spatial learning and memory in the Barnes maze task in rodents-methodological consideration, Naunyn. SchmiedebergsArch. Pharmacol 392 (2019) 1–18, 10.1007/s00210-018-1589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Illouz T, Madar R, Okun E, A modified Barnes maze for an accurate assessment of spatial learning in mice, J. Neurosci. Methods 334 (2020), 108579, 10.1016/j.jneumeth.2020.108579. [DOI] [PubMed] [Google Scholar]
- [57].Lucki I, Dalvi A, Mayorga AJ, Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice, Psychopharmacology 155 (2001) 315–322, 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- [58].Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C, Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal, Biol. Psychiatry 64 (2008) 293–301, 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- [59].Smolinsky AN, Bergner CL, LaPorte JL, Kalueff AV, Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression, in: Gould TD (Ed.), Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests, Humana Press, Totowa, NJ, 2009, pp. 21–36, 10.1007/978-1-60761-303-9_2. [DOI] [Google Scholar]
- [60].Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD, The tail suspension test, J. Vis. Exp (2012), e3769, 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lister RG, The use of a plus-maze to measure anxiety in the mouse, Psychopharmacology 92 (1987) 180–185, 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- [62].Fappiano CM, Baraniuk JN, Gulf War illness symptom severity and onset: a cross-sectional survey, Mil. Med 185 (2020) e1120–e1127, 10.1093/milmed/usz471. [DOI] [PubMed] [Google Scholar]
- [63].Dabisch PA, Davis EA, Horsmon MS, Mioduszewski RJ, Development of miotic cross-tolerance between pyridostigmine and sarin vapor, J. Ocul. Pharmacol. Ther. 22 (2006) 323–332, 10.1089/jop.2006.22.323. [DOI] [PubMed] [Google Scholar]
- [64].Meyer HG, Lukey BJ, Gepp RT, Corpuz RP, Lieske CN, A radioimmunoassay for pyridostigmine, J. Pharmacol. Exp. Ther. 247 (1988) 432–438. https://www.ncbi.nlm.nih.gov/pubmed/3183945. [PubMed] [Google Scholar]
- [65].Li B, Mahan CM, Kang HK, Eisen SA, Engel CC, Longitudinal health study of US 1991 Gulf War veterans: changes in health status at 10-year follow-up, Am. J. Epidemiol. 174 (2011) 761–768, 10.1093/aje/kwr154. [DOI] [PubMed] [Google Scholar]
- [66].Bose D, Saha P, Mondal A, Fanelli B, Seth RK, Janulewicz P, Sullivan K, Lasley S, Horner R, Colwell RR, Shetty AK, Klimas N, Chatterjee S, Obesity worsens gulf war illness symptom persistence pathology by linking altered gut microbiome species to long-term gastrointestinal, hepatic, and neuronal inflammation in a mouse model 12 (2020) 2764, 10.3390/nu12092764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Angoa-Pérez M, Zagorac B, Francescutti DM, Winters AD, Greenberg JM, Ahmad MM, Manning SD, Gulbransen BD, Theis KR, Kuhn DM, Sci. Rep. 10 (2020) 9529, 10.1038/s41598-020-66833-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kelsall HL, McKenzie DP, Sim MR, Leder K, Forbes AB, Dwyer T, Physical, psychological, and functional comorbidities of multisymptom illness in Australian male veterans of the 1991 Gulf War, Am. J. Epidemiol. 170 (2009) 1048–1056, 10.1093/aje/kwp238. [DOI] [PubMed] [Google Scholar]
- [69].Yang Y, Zhao M, Yu X-J, Liu L-Z, He X, Deng J, Zang W-J, Pyridostigmine regulates glucose metabolism and mitochondrial homeostasis to reduce myocardial vulnerability to injury in diabetic mice, Am. J. Physiol. Endocrinol. Metab 317 (2019) E312–E326, 10.1152/ajpendo.00569.2018. [DOI] [PubMed] [Google Scholar]
- [70].Del Rio G, Procopio M, Bondi M, Marrama P, Menozzi R, Oleandri SE, Grottoli S, Maccario M, Velardo A, Ghigo E, Cholinergic enhancement by pyridostigmine increases the insulin response to glucose load in obese patients but not in normal subjects, Int. J. Obes. Relat. Metab. Disord. 21 (1997) 1111–1114, 10.1038/sj.ijo.0800523. [DOI] [PubMed] [Google Scholar]
- [71].Xie H, Lautt WW, Induction of insulin resistance by cholinergic blockade with atropine in the cat, J. Auton. Pharmacol. 15 (1995) 361–369, 10.1111/j.1474-8673.1995.tb00402.x. [DOI] [PubMed] [Google Scholar]
- [72].Takayama S, Legare DJ, Lautt WW, Dose-related atropine-induced insulin resistance: comparing intraportal versus intravenous administration, Proc. West. Pharmacol. Soc. 43 (2000) 33–34. https://www.ncbi.nlm.nih.gov/pubmed/11056951. [PubMed] [Google Scholar]
- [73].Hosey MM, Benovic JL, DebBurman SK, Richardson RM, Multiple mechanisms involving protein phosphorylation are linked to desensitization of muscarinic receptors, Life Sci. 56 (1995) 951–955, 10.1016/0024-3205(95)00033-3. [DOI] [PubMed] [Google Scholar]
- [74].Muthuviveganandavel V, Muthuraman P, Muthu S, Srikumar K, Toxic effects of carbendazim at low dose levels in male rats, J. Toxicol. Sci. 33 (2008) 25–30, 10.2131/jts.33.25. [DOI] [PubMed] [Google Scholar]
- [75].Son H-K, Kim S-A, Kang J-H, Chang Y-S, Park S-K, Lee S-K, Jacobs DR Jr., Lee D-H, Strong associations between low-dose organochlorine pesticides and type 2 diabetes in Korea, Environ. Int 36 (2010) 410–414, 10.1016/j.envint.2010.02.012. [DOI] [PubMed] [Google Scholar]
- [76].Rezg R, Mornagui B, El-Fazaa S, Gharbi N, Organophosphorus pesticides as food chain contaminants and type 2 diabetes: a review, Trends Food Sci. Technol. 21 (2010) 345–357, 10.1016/j.tifs.2010.04.006. [DOI] [Google Scholar]
- [77].Montgomery MP, Kamel F, Saldana TM, Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003. https://academic.oup.com/aje/article-abstract/167/10/1235/231886, 2008. [DOI] [PMC free article] [PubMed]
- [78].Park J, Park SK, Choi Y-H, Environmental pyrethroid exposure and diabetes in U.S. Adults, Environ. Res. 172 (2019) 399–407, 10.1016/j.envres.2018.12.043. [DOI] [PubMed] [Google Scholar]
- [79].Kim J, Park Y, Yoon KS, Clark JM, Park Y, Permethrin alters adipogenesis in 3T3-L1 adipocytes and causes insulin resistance in C2C12 myotubes, J. Biochem. Mol. Toxicol. 28 (2014) 418–424, 10.1002/jbt.21580. [DOI] [PubMed] [Google Scholar]
- [80].Xiao X, Sun Q, Kim Y, Yang S-H, Qi W, Kim D, Yoon KS, Clark JM, Park Y, Exposure to permethrin promotes high fat diet-induced weight gain and insulin resistance in male C57BL/6J mice, Food Chem. Toxicol. 111 (2018) 405–416, 10.1016/j.fct.2017.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang J, Zhu Y, Cai X, Yu J, Yang X, Cheng J, Abnormal glucose regulation in pyrethroid pesticide factory workers, Chemosphere 82 (2011) 1080–1082, 10.1016/j.chemosphere.2010.10.065. [DOI] [PubMed] [Google Scholar]
- [82].Lindheimer JB, Stegner AJ, Wylie GR, Klein-Adams JC, Almassi NE, Ninneman JV, Van Riper SM, Dougherty RJ, Falvo MJ, Cook DB, Post-exertional malaise in veterans with gulf war illness, Int. J. Psychophysiol. 147 (2020) 202–212, 10.1016/j.ijpsycho.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reid S, Hotopf M, Hull L, Ismail K, Unwin C, Wessely S, Multiple chemical sensitivity and chronic fatigue syndrome in british gulf war veterans, Am. J. Epidemiol. 153 (2001) 604–609, 10.1093/aje/153.6.604. [DOI] [PubMed] [Google Scholar]
- [84].Ciccone DS, Weissman L, Natelson BH, Chronic fatigue syndrome in male gulf war veterans and civilians: a further test of the single syndrome hypothesis, J. Health Psychol. 13 (2008) 529–536, 10.1177/1359105308088525. [DOI] [PubMed] [Google Scholar]
- [85].Rayhan RU, Stevens BW, Raksit MP, Ripple JA, Timbol CR, Adewuyi O, VanMeter JW, Baraniuk JN, Exercise challenge in gulf war illness reveals two subgroups with altered brain structure and function, PLoS One. 8 (2013), e63903, 10.1371/journal.pone.0063903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wan J-J, Qin Z, Wang P-Y, Sun Y, Liu X, Muscle fatigue: general understanding and treatment, Exp. Mol. Med. 49 (2017), e384, 10.1038/emm.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang X, Xu Z, Zhao F, Lin KJ, Foster JB, Xiao T, Kung N, Askwith CC, Bruno JP, Valentini V, Hodgetts KJ, Lin C-LG, Restoring tripartite glutamatergic synapses: a potential therapy for mood and cognitive deficits in Gulf War illness 13 (2020), 100240, 10.1016/j.ynstr.2020.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Richtsfeld M, Yasuhara S, Fink H, Blobner M, Martyn JAJ, Prolonged administration of pyridostigmine impairs neuromuscular function with and without down-regulation of acetylcholine receptors, Anesthesiology 119 (2013) 412–421, 10.1097/ALN.0b013e318291c02e. [DOI] [PubMed] [Google Scholar]
- [89].Yamashita M, Yamamoto T, Tryptophan circuit in fatigue: from blood to brain and cognition, Brain Res. 1675 (2017) 116–126, 10.1016/j.brainres.2017.09.002. [DOI] [PubMed] [Google Scholar]
- [90].Yamashita M, Potential role of neuroactive tryptophan metabolites in central fatigue: establishment of the fatigue circuit, Int. J. Tryptophan Res 13 (2020), 1178646920936279, 10.1177/1178646920936279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dickey B, Madhu LN, Shetty AK, Gulf War illness: mechanisms underlying brain dysfunction and promising therapeutic strategies, Pharmacol. Ther. 220 (2021), 107716, 10.1016/j.pharmthera.2020.107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Janulewicz PA, Krengel MH, Maule A, White RF, Cirillo J, Sisson E, Heeren T, Sullivan K, Neuropsychological characteristics of Gulf War illness: a meta-analysis, PLoS One. 12 (2017), e0177121, 10.1371/journal.pone.0177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Baarendse PJJ, van Grootheest G, Jansen RF, Pieneman AW, Ogren SO, Verhage M, Stiedl O, Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice, Hippocampus 18 (2008) 11–19, 10.1002/hipo.20356. [DOI] [PubMed] [Google Scholar]
- [94].Burwell RD, Saddoris MP, Bucci DJ, Wiig KA, Corticohippocampal contributions to spatial and contextual learning, J. Neurosci. 24 (2004) 3826–3836, 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li X, Spence JS, Buhner DM, Hart J Jr., Cullum CM, Biggs MM, Hester AL, Odegard TN, Carmack PS, Briggs RW, Haley RW, Hippocampal dysfunction in gulf war veterans: investigation with ASL perfusion MR imaging and physostigmine challenge, Radiology 261 (2011) 218–225, 10.1148/radiol.11101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Weiner MW, Schuff N, Neylan TC, Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms, Biol. Psychiatry 69 (2011) 541–548, 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Christova P, James LM, Carpenter AF, Lewis SM, Johnson RA, Engdahl BE, Georgopoulos AP, Gulf war illness: C-reactive protein is associated with reduction of the volume of hippocampus and decreased fractional anisotropy of the fornix 5 (2020). https://www.jneurology.com/articles/gulf-war-illness-c-reactive-protein-is-associated-with-reduction-of-the-volume-of-hippocampus-and-decreased-fractional-anisotropy-of-the-fornix.pdf.
- [98].Scremin OU, Shih T-M, Huynh L, Roch M, Booth R, Jenden DJ, Delayed neurologic and behavioral effects of subtoxic doses of cholinesterase inhibitors, J. Pharmacol. Exp. Ther. 304 (2003) 1111–1119, 10.1124/jpet.102.044818. [DOI] [PubMed] [Google Scholar]
- [99].Barker GRI, Warburton EC, When is the hippocampus involved in recognition memory? J. Neurosci. 31 (2011) 10721–10731, 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdóttir HN, Stackman RW Jr., The rodent hippocampus is essential for nonspatial object memory, Curr. Biol. 23 (2013) 1685–1690, 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK, Object location and object recognition memory impairments, motivation deficits and depression in a model of gulf war illness, Front. Behav. Neurosci. 8 (2014) 78, 10.3389/fnbeh.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hubbard NA, Hutchison JL, Motes MA, Shokri-Kojori E, Bennett IJ, Brigante RM, Haley RW, Rypma B, Central executive dysfunction and deferred prefrontal processing in veterans with Gulf War illness, Clin. Psychol. Sci. 2 (2014) 319–327, 10.1177/2167702613506580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].White RF, Proctor SP, Heeren T, Wolfe J, Krengel M, Vasterling J, Lindem K, Heaton KJ, Sutker P, Ozonoff DM, Neuropsychological function in Gulf War veterans: relationships to self-reported toxicant exposures, Am. J. Ind. Med. 40 (2001) 42–54, 10.1002/ajim.1070. [DOI] [PubMed] [Google Scholar]
- [104].Joshi U, Evans JE, Joseph R, Emmerich T, Saltiel N, Lungmus C, Oberlin S, Langlois H, Ojo J, Mouzon B, Paris D, Mullan M, Jin C, Klimas N, Sullivan K, Crawford F, Abdullah L, Sci. Rep. 9 (2019) 11011, 10.1038/s41598-019-46255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zakirova Z, Tweed M, Crynen G, Reed J, Abdullah L, Nissanka N, Mullan M, Mullan MJ, Mathura V, Crawford F, Ait-Ghezala G, PLoS One. 10 (2015), e0119579, 10.1371/journal.pone.0119579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zakirova Z, Crynen G, Hassan S, Abdullah L, Horne L, Mathura V, Crawford F, Ait-Ghezala G, A chronic longitudinal characterization of neurobehavioral and neuropathological cognitive impairment in a mouse model of gulf war agent exposure, Front. Integr. Neurosci. 9 (2015) 71, 10.3389/fnint.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Abdullah L, Evans JE, Joshi U, Crynen G, Reed J, Mouzon B, Baumann S, Montague H, Zakirova Z, Emmerich T, Bachmeier C, Klimas N, Sullivan K, Mullan M, Ait-Ghezala G, Crawford F, Translational potential of long-term decreases in mitochondrial lipids in a mouse model of Gulf War illness, Toxicology 372 (2016) 22–33, 10.1016/j.tox.2016.10.012. [DOI] [PubMed] [Google Scholar]
- [108].Noorbakhshnia M, Karimi-Zandi L, Portulaca oleracea L prevents lipopolysaccharide-induced passive avoidance learning and memory and TNF-α impairments in hippocampus of rat, Physiol. Behav. 169 (2017) 69–73, 10.1016/j.physbeh.2016.11.027. [DOI] [PubMed] [Google Scholar]
- [109].Binns JH, Barlow C, Bloom FE, Clauw DJ, Nettleman MD, Gulf War Illness and the Health of Gulf War Veterans, 2008. https://www.researchgate.net/publication/235042747_Gulf_War_Illness_and_the_Health_of_Gulf_War_Veterans. (Accessed 28 February 2021).
- [110].Mineur YS, Picciotto MR, The role of acetylcholine in negative encoding bias: too much of a good thing? Eur. J. Neurosci. 53 (2021) 114–125, 10.1111/ejn.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen L, Faas GC, Ferando I, Mody I, Novel insights into the behavioral analysis of mice subjected to the forced-swim test, Transl. Psychiatry 5 (2015), e551, 10.1038/tp.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Willner P, Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS, Neuropsychobiology 52 (2005) 90–110, 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- [113].Blore JD, Sim MR, Forbes AB, Creamer MC, Kelsall HL, Depression in Gulf War veterans: a systematic review and meta-analysis, Psychol. Med 45 (2015) 1565–1580, 10.1017/S0033291714001913. [DOI] [PubMed] [Google Scholar]
- [114].Kodali M, Hattiangady B, Shetty GA, Bates A, Shuai B, Shetty AK, Curcumin treatment leads to better cognitive and mood function in a model of Gulf War illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus, Brain Behav. Immun 69 (2018) 499–514, 10.1016/j.bbi.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.