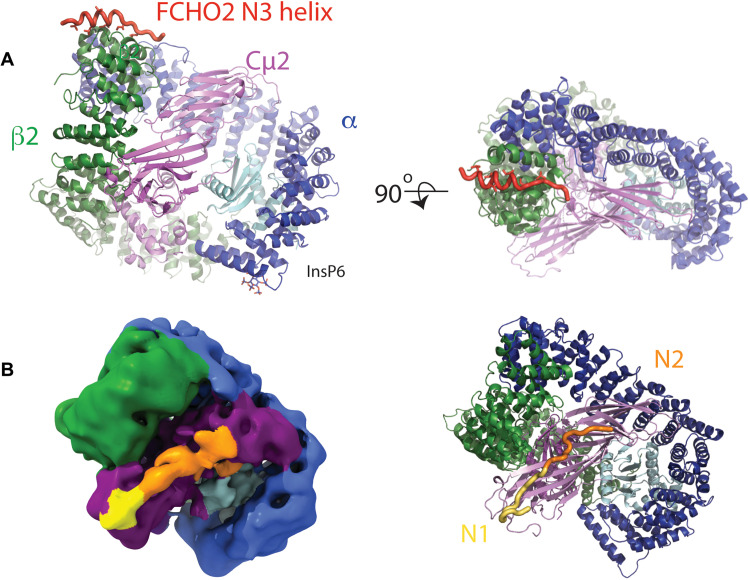
Fig. 5. FCHO linker N1, N2, and N3 blocks can bind and activate closed and part-open conformers of AP2.
(A) Structure of AP2:β2FCHO2linker chimera showing the position of the bound N3 helix (red) and InsP6 in orthogonal as indicated. (B) Left: Single-particle EM reconstruction of AP2:μ2FCHO2-N1+N2+N3 chimera highlighting the position of FCHO2-N1+N2 (see N1N2-enriched subclass in fig. S5 and table S6). The map, locally filtered with a global resolution of 7 Å, is colored according to AP2 subunit, the positions of which are derived from a fitted closed AP2 core (PDB 2vgl), and the approximate locations of N1 (yellow) and N2 (orange) are also indicated. N1 is persistent in all reconstructions of AP2:μ2FCHO2-N1+N2+N3 chimera, whereas N2 appears to be less fixed (fig. S5): N3 is not seen in any single-particle reconstructions. The position of N1 is in agreement with x-ray crystallographic structures (Fig. 4). N2 appears to extend from the N1 density associated with the Cμ2 BR3 site across the broad, positively charged BR4 surface patch of Cμ2. Right: Ribbon representation of AP2 in the same orientation as the left panel with FCHO linker shown in worm representation.