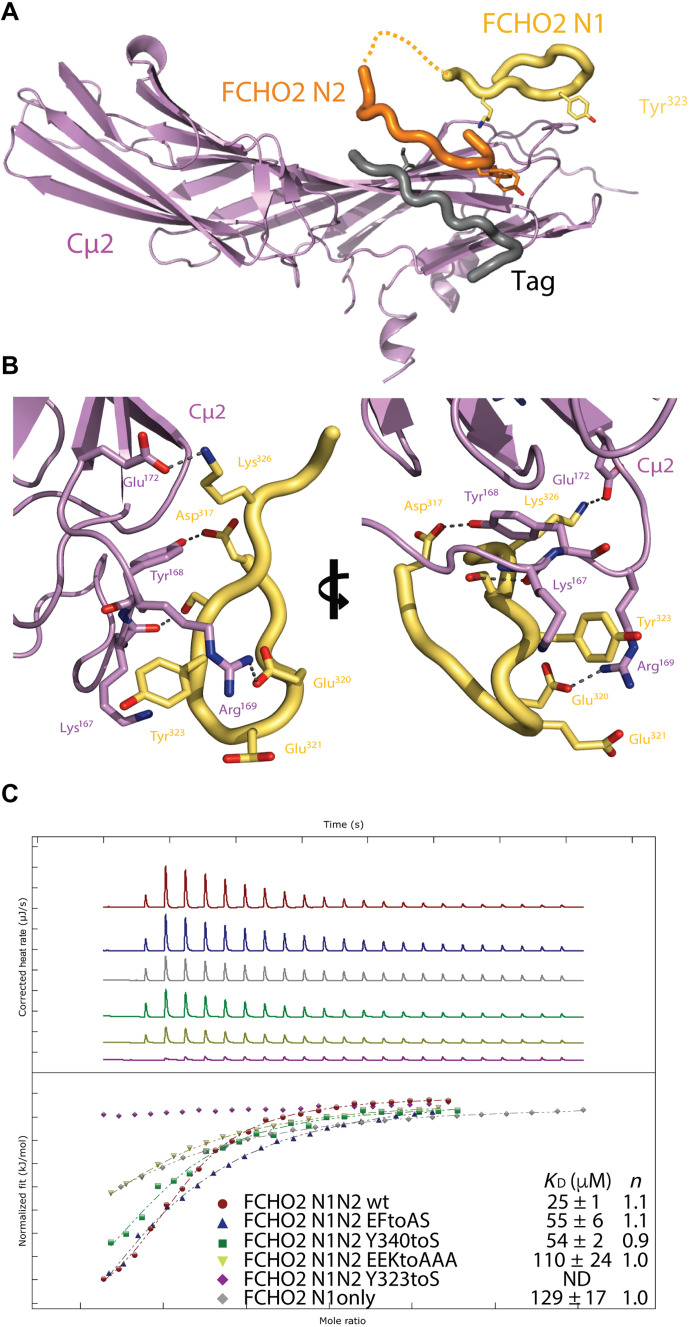
Fig. 6. Binding of N1 to Cμ2.
Overall positioning (A) and molecular details (B) of the N1 block bound to isolated Cμ2, allowing better definition of N1’s binding to Cμ2 in whole AP2. The position of N2 and an affinity tag (gray) occupying the YxxΦ-binding site are also shown. The N1 interaction occurs mainly via complementary charged interactions between Asp318, Glu320, and Glu321 and the Cμ2 BR3 PtdIns(4,5)P2-binding site containing Lys167, Tyr168, and Arg169. (C) Confirmation of N1 binding site on Cμ2 by ITC using structure-directed mutants. E321A+E322A+K326A reduces binding ~5-fold from ~25 μM, and mutating Y323S renders binding unmeasurably weak and not determinable (ND). Mutations that fall outside the binding interface, Y340S and E336A+F339S, have little effect in binding. Deleting N2 also reduces binding fivefold.