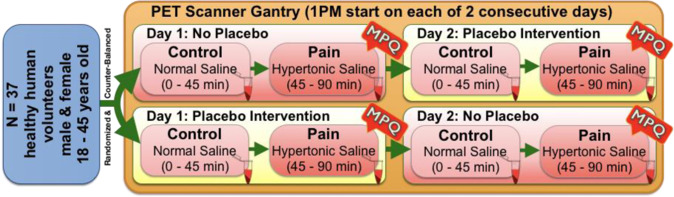
Fig. 1. Research paradigm.
The sample derived from n = 37 healthy volunteers (12 males, 25 females). Once enrolled, volunteers were randomized and counterbalanced to 1 of 2 intervention orders as illustrated by the green double arrows on the left. As such, a given subject was randomized to either Order #1: No Placebo on Day 1 and Placebo Intervention on day 2 (see top row of diagram) or Order #2: Placebo intervention on Day 1 and No Placebo on Day 2 (bottom row of diagram). Regarding Placebo/NoPlacebo administration, subjects were told that on one of the two scanning days they will receive an intravenous dose of “an agent that may increase the body’s ability to counter pain” and that on the other scanning day they will receive no intervention (e.g., an inert substance). After completion of the studies, the volunteers were informed that the agent was a placebo, an inactive agent.