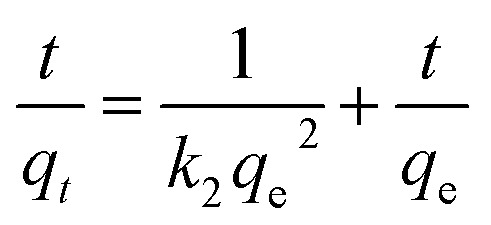| Pseudo-first order |
ln(qe − qt) = ln qe − k1t
|
q
t
(mg g−1): removed amount of MO at time t
|
53
|
|
q
e (mg g−1): equilibrium sorption uptake |
|
K
1 (g mg−1 min−1): rate constant of the first-order adsorption |
|
k
1: −slope |
|
q
e (cal): EXP(intercept)
|
| Pseudo-second order |
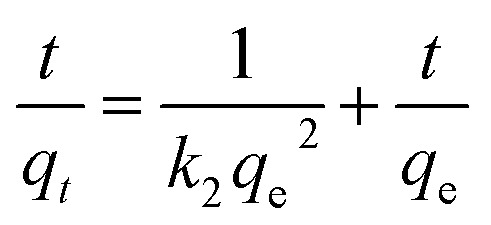
|
q
t
(mg g−1): removed amount of MO at time t
|
54
|
|
q
e (mg g−1): equilibrium sorption uptake |
|
K
2 (g mg−1 min−1): rate constant of the second-order adsorption |
|
q
e (cal) = 1/slope |
|
k
2 = (slope) × 2/intercept |
| Intra-particle diffusion |
q
t
= kpt1/2 + C
|
q
t
(mg g−1): removed amount of MO at time t
|
55
|
|
K
p (mg g−1 min−0.5): intra-particle diffusion rate constant |
|
C (mg g−1): intercept of the line which reflects the thickness of the boundary layer |
|
k
p = slope |
|
C = intercept |