Substrate scope of CsNF-catalyzed Knoevenagel condensationa.
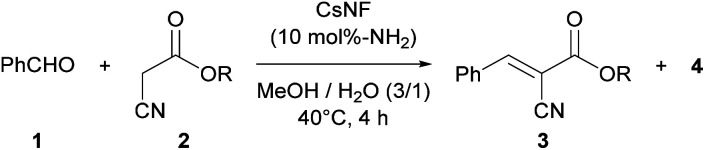
| ||||
|---|---|---|---|---|
| Entry | 2 | Product | % Yieldb (3/4) | |
| CsNF | n-Hexylamine | |||
| 1 |
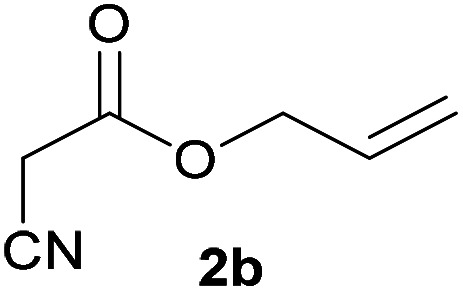
|
3b | 76/n.dc | 10/81 |
| 2 |
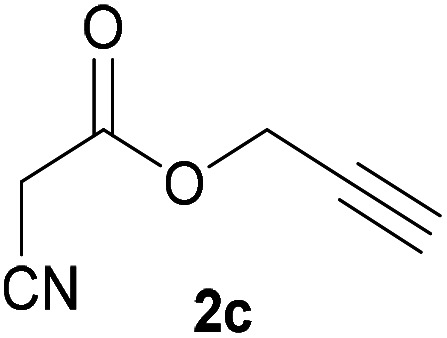
|
3c | 86/n.dc | n.dc/91 |
| 3d |
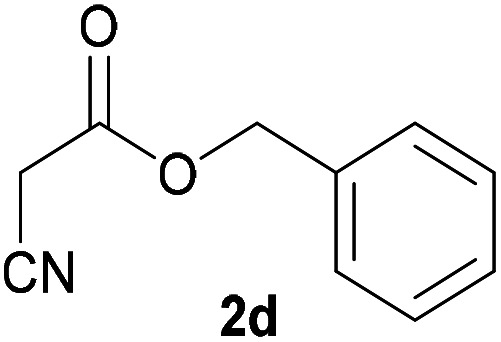
|
3d | 53/n.dc | n.dc/87 |
| 4 |
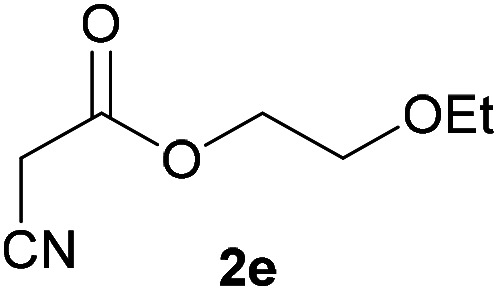
|
3e | 75/2 | 11/79 |
| 5 |
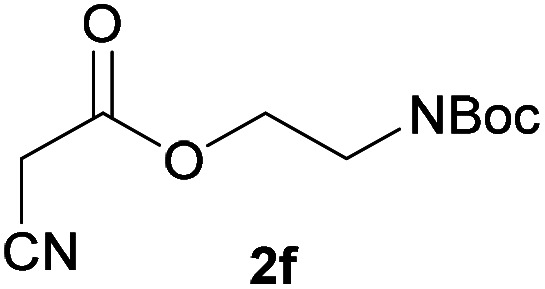
|
3f | 76/2 | 5/86 |
| 6 |
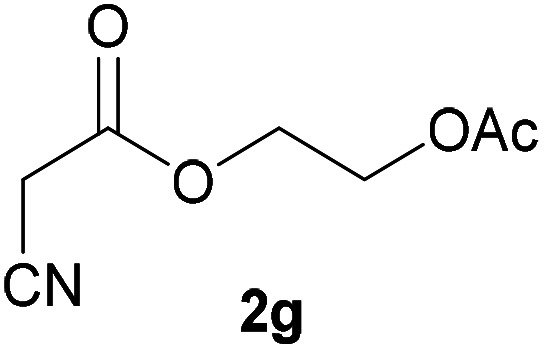
|
3g | 79/2 | 1/91 |
Unless otherwise noted, the reaction was carried out using 1 (2.0 mmol, 203.3 μL) and 2 (1.0 mmol, 106.2 μL) in MeOH (22.5 mL)/H2O (7.5 mL).
Determined by 1H NMR analysis using CH2Br2 as an internal standard.
Not detected.
The reaction was carried out for 24 h.
