Figure 3. Disruption of tissue mechanics in breast cancer.
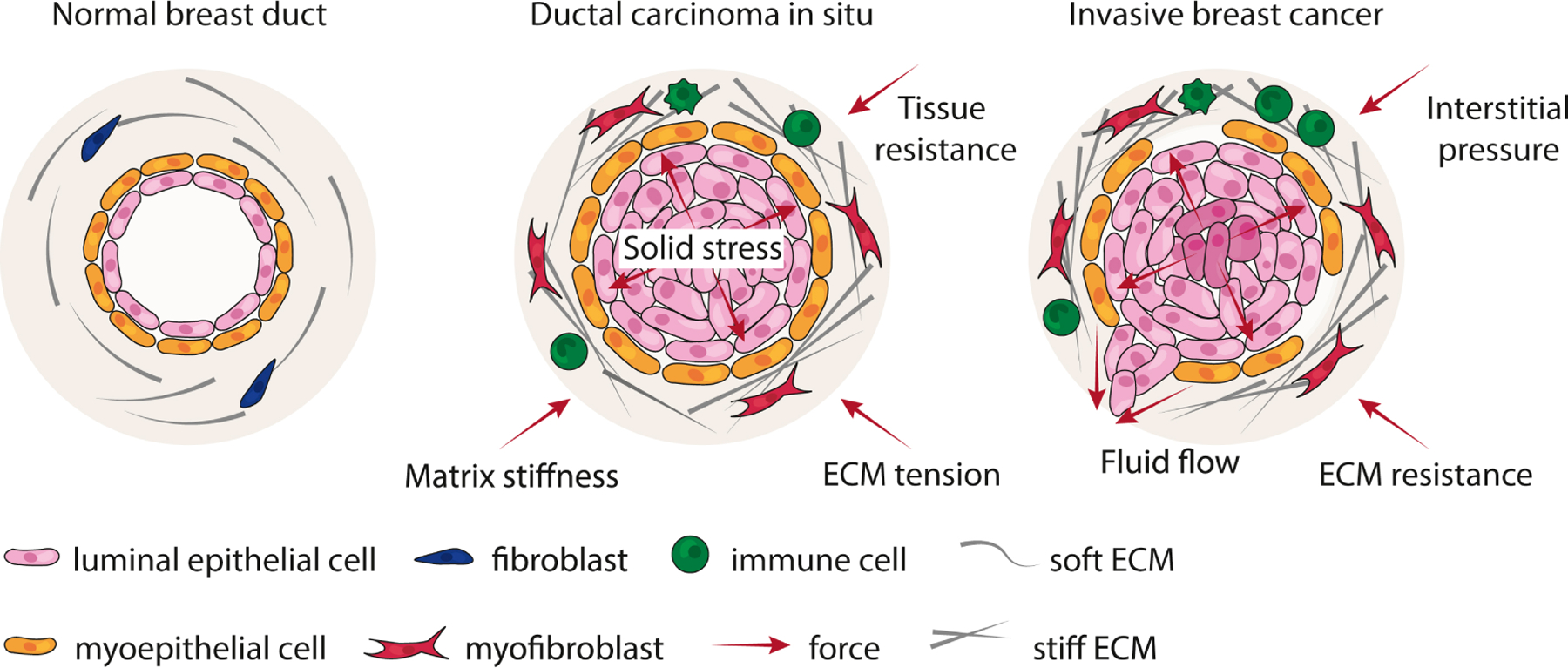
In the normal breast, forces between the epithelium and stroma maintain a state of tensional homeostasis. Normal ducts are surrounded by a compliant and soft ECM. In ductal carcinoma in situ (DCIS), epithelial cells proliferate within the lumen of the duct, exerting an outward projective compressive force on the adjacent myoepithelium and basement membrane (solid stress). These forces are countered by an inward projecting resistance force from the ECM. Accompanying DCIS progression is the remodeling, crosslinking and stiffening of the ECM by myofibroblasts and immune infiltrate. In parallel, interstitial pressure accumulates due to impaired lymphatic drainage and vessel compression. Together, these forces can generate regions of hypoxia within a tumor, which can induce epithelial-to-mesenchymal or stem-like transition in tumor cells. Neoplastic epithelial cells exhibit modified tensional homeostasis and respond to these accumulating forces. At some point, the myoepithelial-basement membrane barrier is breached, and linearized and stiffened ECM tracks surrounding ducts facilitate tumor cell invasion and metastasis.
