NMR spectroscopic data of compounds 1-3 and cannabitriol-C3 (in CDCl3).
| No. | 1 | 2 | 3 | Cannabitriol-C3 | ||||
|---|---|---|---|---|---|---|---|---|
| δ H (J in Hz) | δ C | δ H (J in Hz) | δ C | δ H (J in Hz) | δ C | δ H (J in Hz) | δ C | |
| 1 | 153.4 | 153.4 | 155.4 | 152.2 | ||||
| 2 | 6.35, d (1.6) | 111.2 | 6.36, d (1.6) | 111.1 | 6.17, d (1.4) | 109.4 | 6.34, d (1.6) | 110.9 |
| 3 | 144.8 | 145.1 | 144.5 | 144.5 | ||||
| 4 | 6.28, d (1.6) | 108.7 | 6.28, d (1.6) | 108.6 | 5.95, d (1.4) | 108.3 | 6.30, d (1.6) | 109.3 |
| 4a | 153.5 | 153.5 | 153.7 | 153.7 | ||||
| 6 | 76.3 | 76.3 | 75.7 | 76.6 | ||||
| 6a | 138.1 | 138.0 | 1.49, m | 46.9 | 136.1 | |||
| 7 | 2.20, dd (19.3, 6.2) | 22.1 | 2.20, dd (19.3, 6.0) | 22.1 | 1.35, m | 17.1 | 2.14, dt (19.0, 4.7) | 22.6 |
| 2.45, m | 2.46, m | 1.67, m | 2.39, m | |||||
| 8 | 1.75, m | 30.9 | 1.75, m | 30.9 | 1.81, ddd (14.5, 13.0, 4.6) | 29.7 | 1.78, m | 29.1 |
| 1.89, dd (14.2, 7.3) | 1.89, dd (14.2, 7.3) | 2.06, dt (14.5, 3.2) | ||||||
| 9 | 70.1 | 70.1 | 61.6 | 70.5 | ||||
| 10 | 4.27, br s | 77.6 | 4.27, br s | 77.6 | 3.87, s | 66.3 | 4.19, br s | 72.4 |
| 10a | 117.8 | 117.8 | 68.7 | 122.2 | ||||
| 10b | 108.4 | 108.3 | 109.0 | 109.0 | ||||
| 11 | 1.30, s | 23.7 | 1.30, s | 23.7 | 1.39, s | 25.8 | 1.23, s | 23.5 |
| 12 | 1.50, s | 25.4 | 1.50, s | 25.4 | 1.40, s | 27.7 | 1.45, s | 25.2 |
| 13 | 1.41, s | 26.3 | 1.41, s | 26.3 | 1.45, s | 22.6 | 1.39, s | 25.1 |
| 1′ | 2.45, m | 37.7 | 2.46, m | 35.6 | 2.27, m | 37.6 | 2.45, t (7.2) | 37.7 |
| 2′ | 1.60, m | 23.8 | 1.58, m | 30.4 | 1.48, m | 23.7 | 1.60, m | 23.9 |
| 3′ | 0.93, t (7.3) | 13.9 | 1.30, m | 31.5 | 0.87, t (7.3) | 14.0 | 0.92, t (7.3) | 13.8 |
| 4′ | 1.31, m | 22.5 | ||||||
| 5′ | 0.88, t (6.9) | 14.0 | ||||||
| 1-OH | 9.40, br s | 9.40, br s | 7.38, br s | 8.57, br s | ||||
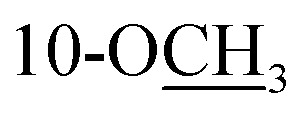
|
3.34, s | 51.9 | 3.34, s | 51.9 | ||||
