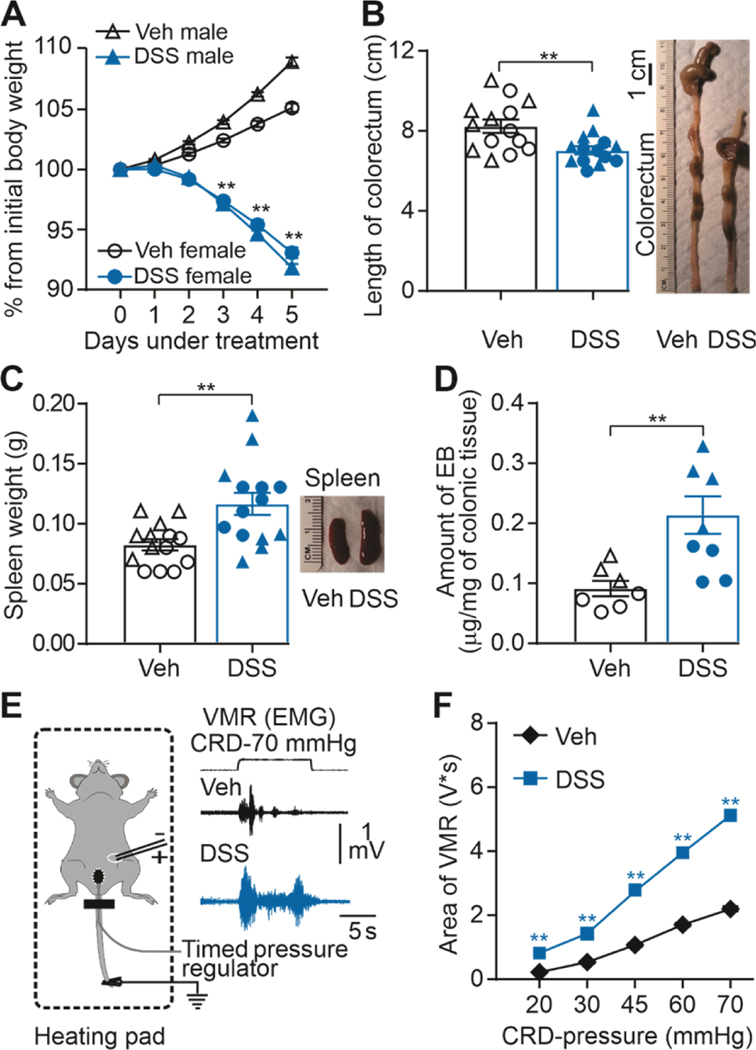
Figure 1. Dextran sodium sulfate (DSS) administration produces colon inflammation.
A) Percentage change from initial body weight during DSS or sterile water (Veh) treatment. Significant differences in body weight emerge at day three of treatment in male and female mice (**P < 0.01, three-way ANOVA, n = 49 and n = 37 male and female respectively). B) Pooled colorectum length data from fourteen DSS-treated and vehicle-treated mice (**P < 0.01, two-way ANOVA). At the right, representative colorectum imagen from DSS-treated and vehicle-treated mice. C) Pooled spleen weight data from fourteen DSS-treated and vehicle-treated mice (respectively.**P < 0.01; two-way ANOVA). D) Pooled EB in DSS-treated and vehicle-treated mice (**P < 0.01, n = 7 per group; two-way ANOVA). E) Experimental setting for the recording of the visceromotor response (VMR) to colorectum distention (CRD). F) Visceromotor responses- area presented as stimulus-response functions reveal significant colon hypersensitivity after DSS treatment compared with sterile- water treatment. **p < 0.01; two-way ANOVA; n=86 mice/group. Of note, in this and subsequent figures, when data from individual mice are plotted, males are represented by triangles and females by circles, with open symbols used for vehicle treated mice and closed symbols used for DSS-treated mice.