Abstract
A total of 84 volatile aroma components were determined in the 9 samples of sugarcane to non-centrifugal sugar (NCS), including 15 alcohols, 12 aldehydes, 10 ketones, 17 carboxylic acids, 11 pyrazines, 7 phenols, 3 esters, 3 hydrocarbons, and 2 sulfur compounds. Of these compounds, 10 were with high flavor dilution (FD) factors based on the aroma extract dilution analysis (AEDA). 4-Hydroxy-2,5-dimethyl-3(2H)furanone exhibited the highest FD factor of 2187, followed by (E)-2-nonenal, 2-hydroxy-3-methyl-2-cyclopentene-1-one, and 4-allyl-2,6-dimethoxyphenol with a FD factor of 729. The odor compounds showed no significant change and were similar to that of sugarcane during the first four steps in the production of non-centrifugal cane sugar. In the middle three stages, the heating slightly affected the aroma composition. Additionally, a prolonged period of high-temperature heating, lead to the production of the Maillard reaction products, such as pyrazines, pyrroles, and furans, differentiating the step to be unique from the previous seven stages. However, the content of the NCS odorants was significantly reduced due to the loss of odor compounds during the drying process.
84 volatile aroma components were determined in 9 samples of sugarcane to non-centrifugal sugar (NCS), including 15 alcohols, 12 aldehydes, 10 ketones, 17 carboxylic acids, 11 pyrazines, 7 phenols, 3 esters, 3 hydrocarbons, and 2 sulfur compounds.
1. Introduction
Sugarcane belongs to several species of tall perennial grasses of the genus Saccharum and is characteristically tropical or subtropical with a high concentration in South Asia. It is an important economic crop and widely grown as a commercial crop primarily in Brazil, USA, Mexico, India, China, Thailand, and Australia.1 Sugarcane is a rich source of sugar and often used for sugar production as it can store high concentrations of sugar in the stem.1 The products derived from sugarcane include sugar, molasses, rum, bagasse, and ethanol.1,2 China is the 3rd largest sugar producing country in the world after Brazil and India.3 In the recent decade, sugarcane contributed more than 90% of the total sugar production.3 The extraction of juice by squeezing the sugarcane through the mill produces a large amount of bagasse. However, a small amount of sugar still remains in the bagasse. Therefore, it is necessary to mill the bagasse again after spraying hot water on the bagasse.4 The obtained juice is transported to the purification system with the addition of calcium oxide and sodium bicarbonate to accelerate the precipitation and reduce acrylamide production,5–7 followed by water evaporation, juice concentration to obtain the syrup. Finally, the syrup is crystallized by mechanical agitation or manual stirring between 115 to 120 °C and molding to give a solid shape (Fig. 2).4,5
Fig. 2. Sugarcane.
NCS is characterized by several phenolic and flavonoid compounds having antioxidant properties and therefore, exerts potential benefits for the organisms,8–14 and the enzymatic browning of phenol affects the color of sugarcane.15 Additionally, NCS exerts immune activity, cytoprotective effects, anti-caries, and anti-cancer properties.16,17 Asikin et al.18 studied the physicochemical properties of NCS during the storage, and found the color of NCS to be darker. He also observed increased water content and water activity, as well as reduced glucose and fructose content due to their participation in the Maillard reaction. Similarly, Huang et al.19 analyzed the odor components of NCS and determined acetaldehyde, 2-methylbutyraldehyde, 3-methylbutyraldehyde, 2,6-dimethylpyrazine, nonanal, 2,6-diethylpyrazine, 2,3,5-trimethylpyrazine, furfural, 2,3-dimethylpyrazine, decanal, and 2-acetylpyrrole to be the main components based on their relative concentration. Juliana et al.20 extracted a total of 6 odor compounds from NCS beverage using a mixture of diethyl ether–pentane (1 : 1, w/w) as the solvent through simultaneous steam distillation-solvent extraction. Of the 6 components, 2-methylpyrazine was the key aroma compound of this beverage. Takahashi et al.21 prepared 2 types of NCS from the whole stalk (W-NCS) and separated pith of sugarcane (P-NCS) and observed no significant difference between the sugars and minerals' compositions of the 2 types of sugar. Therefore, their taste profile was very similar, but the aroma intensity of P-NCS was weaker than that of W-NCS.
NCS exhibits a unique aroma that makes it different from refined sugar.5 However, not a single study on the alternation of the volatile flavor compositions of sugarcane during the processing of sugarcane to obtain NCS has been reported to date. Therefore, the present study aimed at revealing the changes of flavor compounds during the production of NCS from sugarcane, and providing possible guidance for the production of NCS. The flavor was analyzed in 9 samples taken from the industrial line (Fig. 1).
Fig. 1. Production diagram from sugarcane to NCS.
2. Materials and methods
2.1. Materials
The 9 samples were obtained from a sugar factory named YUEBEI Brown Sugar in Qingyuan, Guangdong province, China. Samples were stored at a −25 °C refrigerator prior to the analysis. The 9 samples used in this experiment are listed in Table 1.
9 samples used in the experimenta.
| No. | Sample name | pH | °Brix |
|---|---|---|---|
| 1 | Sugarcane | — | — |
| 2 | Juice extraction | 5.4 | 18.26 |
| 3 | Juice extraction mixture | 5.4 | 10.87 |
| 4 | Juice extraction mixture with calcium oxide and sodium bicarbonate | 6.4 | 15.57 |
| 5 | Clean juice | 6.9 | 15.57 |
| 6 | Impurities | 7.7 | — |
| 7 | Concentrated juice | 6 | 69.12 |
| 8 | Syrup | — | — |
| 9 | NCS | — | — |
“—” means not detected or cannot be detected.
2.2. Standards and reagents
Chemicals (purity > 99%), such as ethyl ether, n-hexane, and anhydrous sodium sulfate were purchased from Lab Gou e-mall (Beijing, China). Other chemicals, including 2-methyl-3-heptanone, n-alkanes (C7–C30) were purchased from Sigma-Aldrich (St. Louis, U.S.A.). Nitrogen gas (99.9992% purity) was obtained from Beijing AP BAIF Gases Industry Co., Ltd. (Beijing, China) and the liquid nitrogen was obtained from XianHeyu Trading Co., Ltd. (Beijing, China).
2.3. Volatile aroma components analysis
2.3.1. Odor compounds extraction from sugarcane
Sugarcane was cut into small bricks, frozen by liquid nitrogen, and then, ground into powders. The volatile aroma components of the sugarcane were extracted using an organic solvent followed by a liquid–liquid extraction (LLE) method.22 In brief, 100.00 g of sample, 50 mL of diethyl ether, 50 mL of dichloromethane, and 5 μL of internal standard 2-methylheptan-3-one (81.6 mg mL−1) were mixed in a triangular flask, and was stirred for 10 minutes with a magnet (3 cm × 8 mm diameter) at 1000 rpm. After centrifugation (Hitachi, Japan) for 30 minutes at 10 000 rpm, the extract containing volatile aroma components were separated by a straw. Afterward, the extract was dehydrated over 150.0 g anhydrous sodium sulfate for 12 h at 4 °C and filtered using a filter paper. The volume was then reduced to 100 μL under a gentle nitrogen stream, and the obtained volatile aroma component extract was stored at −25 °C until further assayed.
2.3.2. Odor compounds extraction from liquid sample
For liquid sample preparation, 100 mL sample, 50 mL of diethyl ether, 50 mL of dichloromethane, and 5 μL of internal standard 2-methylheptan-3-one (81.6 mg mL−1) were mixed in a triangular flask and then stirred for 10 minutes with a magnet at 1000 rpm. The extraction process was the same as described in the 2.3.1 Section.
2.3.3. Odor compounds extraction from NCS
For NCS sample preparation, 100.00 g NCS was dissolved in 100 mL of distilled water. Then, all NCS aqueous solution sample, 50 mL of diethyl ether, 50 mL of dichloromethane, and 5 μL of internal standard 2-methylheptan-3-one were mixed in a triangular flask, and stirred for 10 minutes with a magnet at 1000 rpm. The extraction process was the same as described in the 2.3.1 Section.
2.3.4. Gas chromatography-olfactometry-mass spectrometry (GC-O-MS)
An Agilent 7890A gas chromatography (GC) coupled with an Agilent 5977B mass spectrograph (MS) and a sniffing port (Gerstel, Germany) was employed to analyze the volatile aroma component profiles. The aroma extract (1 μL) was injected into a DB-Wax column (30 m × 0.25 mm i.d., film thickness 0.25 μm, Agilent J&W) at a splitless mode, and the flow rate of the helium carrier gas was maintained 1.7 mL min−1. The temperature of the oven was initially set at 40°, raised to 100 °C at a rate of 4 °C min−1, and then raised to 200 °C at a rate of 3 °C min−1 for 5 min, and then raised to 230 °C at a rate of 3 °C min−1 and held constant at 230 °C for 10 min. Both the transmission and exit temperature of ODP was 235 °C. For MS detection, the interface and ion source was programmed at 250 °C and 230 °C, respectively, whereas the electron-impact ionization was at 70 eV, acquisition range (m/z) at 35–350 amu, and the scan rate at 1.77 scans per s.
2.3.5. Qualitative analysis
The peak identification was performed by comparing the MS fragmentation patterns with the National Institute of Standards and Technology (NIST) MS Spectral Library, Version 2017. Further identification was confirmed by comparing their flavors and their linear retention indices (RIs) towards a homologous series of n-alkanes (C7–C30). The resulting peak was calibrated by area of the internal standard, and the content of aroma compounds was expressed as nanograms per gram sample.23 All the analyses were carried out in triplicate.
2.3.6. Aroma extraction dilution analysis (AEDA)
Aroma Extraction Dilution Analysis (AEDA) was employed to characterize the contribution of odorants to the overall aroma.24 For AEDA, aroma extracts were stepwise-diluted with the mixture of dichloromethane and diethyl ether (1 : 1, v/v) in a 1 : 2 ratio. The sensory evaluation was performed via GC-O-MS by three panelists (two females and one male). Detection of an odor at the sniffing port sniffed by at least two panelists was considered as the availability.
2.4. Statistical analysis
All the experiments were carried out in triplicate, and the data were expressed as the mean ± standard deviation. Statistical analyses were conducted using SIMCA 14.1 (MKS Instruments AB, USA), OriginPro 2020b (OriginLab Corp., Washington, MA, USA) and Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA).
3. Results and discussion
3.1. Volatile aroma components analysis
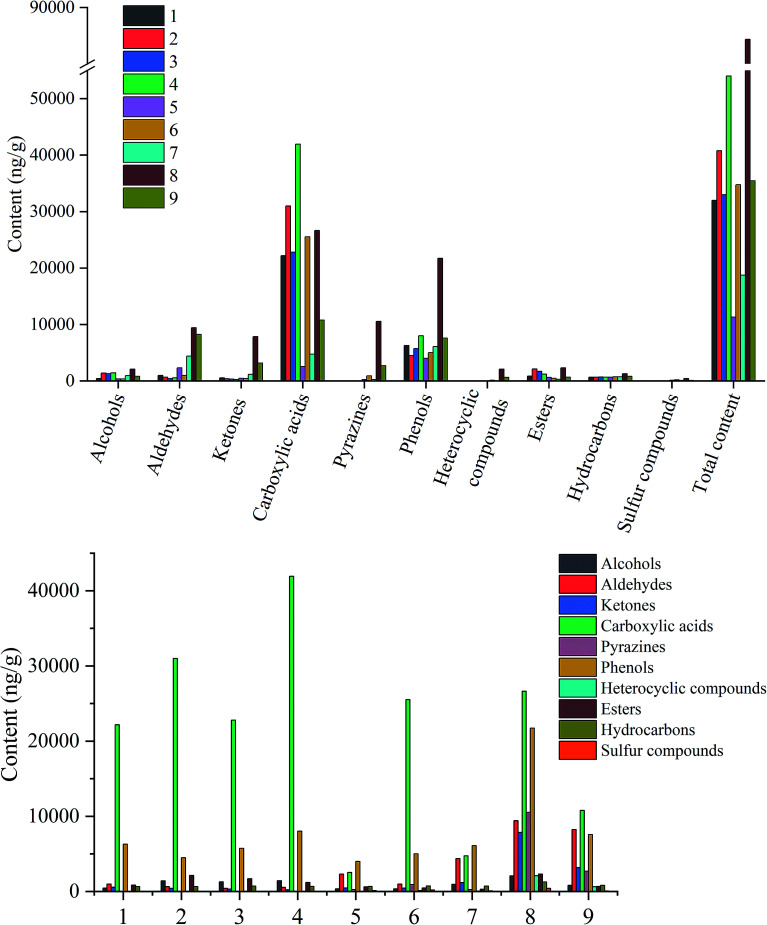
A total of 84 volatile aroma components were found from the evaluated samples, including 15 alcohols, 12 aldehydes, 10 ketones, 17 carboxylic acids, 11 pyrazines, 7 phenols, 3 esters, 3 hydrocarbons, and 2 sulfur compounds (Table 1 and Fig. 3).
Fig. 3. Quantitation and qualitation of volatile aroma components of the samples from sugarcane to non-centrifugal cane sugar.
More than half of the flavor components in sugarcane were found to be acid compounds (22 175.53 ng g−1), followed by phenols (6283.11 ng g−1). These two components, carboxylic acids and phenols, account for 89.04% of the total amount in sugarcane. The other compounds that are available in low contents are aldehydes, esters, hydrocarbons, ketones, alcohols, and heterocyclic compounds (985.59, 842, 652.52, 558.22, 442.56 and 21.94 ng g−1 respectively). However, no pyrazines were detected in sugarcane. This might attribute to the low temperature and low reaction rate of the Maillard reaction.25 Each volatile flavor compounds of sugarcane might contribute to the overall aroma quality and characteristic.18 Accumulating studies indicate that butanoic acid might provide yogurt and papaya aromas, whereas propionic acid provides an acidic characteristic, and hexanoic and octanoic acids provide sweaty or cheesy aromas.18 Green and grass odor are the main aroma characteristics of sugarcane, primarily contributed by 1-hexanol, 2,3-butanediol, hexanal, (E)-2-octenal, nonanal, (E)-2-nonenal, acetoxyacetone, formic acid, and nonanoic acid.18,26 Moreover, benzaldehyde contributes a sweet flavor to the sugarcane.18 A mixture of other volatile components might also complement the complexity of flavor characteristics in NCS, viz. 2-heptanol and 1-octen-3-ol (mushroom), 2-ethyl-1-hexanol (rose, green), phenylethyl alcohol (honey), and phenylacetic acid (honey, flower).
When the juice was squeezed out from the sugar cane, the contents of carboxylic acids, alcohols, esters, and total components increased. The contents of (E)-2-octenal, nonanal, (E,E)-2,4-heptadienal and (E)-2-nonenal significantly increased than that in sugarcane. Similarly, in ester group, the contents of ethyl hexadecanoate and dibutyl phthalate also increased.
When it comes to the juice extraction mixture, almost all compounds decreased, except phenols and hydrocarbons. It may be caused by adding water. During the subsequent extraction process, the total concentration gradually decreased.
In the juice extraction mixture with calcium oxide and sodium bicarbonate, the content of the compounds slightly fluctuated except for carboxylic acids and phenols. This phenomenon might have happened by the bacteria during the addition of calcium oxide and sodium bicarbonate.27
During the clarification process, polyacrylamide adsorbs the impurities and mediates the precipitation of some flavor components, causing the loss of flavor components.5 As a result, the flavor content in the clear juice decreased compared to the mixed juice, and the impurities also contained some flavor components. However, in the high-temperature clarification stage, some changes in the generation of pyrazines and other compounds were observed. For instance, hexanal, 3,5-dimethoxy-4-hydroxybenzaldehyde, 2-hydroxy-3-methyl-2-cyclopentene-1-one, 3-hydroxy-4,4-dimethyldihydro-2(3H)-furanone, 4′-hydroxyacetophenone, 2,6-dimethylpyrazine, 2,5-dimethylpyrazine, 2,3,5-trimethylpyrazine, 2-methoxy-4-acetylphenol, dimethyl sulfoxide, and methyl sulfone were produced during this period. The formation of these compounds might attribute to the maintenance of high temperature for a prolonged time during the clarification process, allowing them to undergo complex biochemical reactions.28 Besides, the clean juice started developing slight nutty, roasted, and caramel characteristics, that might be due to the generation of pyrazines (roasted nut, cocoa, roast beef), 3,5-dimethoxy-4-hydroxybenzaldehyde (sweet, cocoa, nutty), 2-hydroxy-3-methyl-2-cyclopentene-1-one (caramellic) and 3-hydroxy-4,4-dimethyldihydro-2(3H)-furanone (cotton candy). These compounds enrich the overall aroma profile of clean juice and even later CNS.
The clean juice was then transferred to a three-effect evaporator for evaporation and concentration. The temperatures of the first, second and third-effect evaporators were maintained at 105 °C, 105 °C, and 82 °C, respectively until the solid content reached 93%. The time interval for the formation of concentrated juice from clear juice was about 45 min. During this period, the total identified compounds, including alcohols, aldehydes, ketones, carboxylic acids, phenols, heterocyclic compounds, and hydrocarbons, increased significantly. However, a significant reduction was observed in the contents of esters and sulfur compounds with no much change in pyrazines.
After boiling at 123 °C for 1 h, a thick syrup was obtained. The syrup showed semi-solid and semi-fluidity state. At this time, the content of alcohols, aldehydes, ketones, carboxylic acids, pyrazines, phenols, heterocyclic compounds, esters, hydrocarbons, sulfones, and the total odor compounds reached the highest value. Of them, the number of pyrazine compounds increased from 2 to 10, and the contents increased from 255.63 ng g−1 to 10 536.35 ng g−1 due to the Maillard reactions occurring at high temperatures.25 Pyrazines are the most popular products of the Maillard reaction with a low threshold value; thus, they are considered as the important flavor components in the cooked foods and other high sugar products, such as syrups. Methylpyrazines exhibits nutty and popcorn-like aromas, while 2,6-dimethylpyrazine adds a roasted or cooked flavor in foods. They have been shown to be the most dominant aromatic compounds formed by the reaction of l-ascorbic acid with glutamic acid in the presence of water during heating.21
The last sample was the NCS. The content of odor compounds in the NCS reduced due to the loss of a large amount of odor compounds during the final cooling molding and drying process. This result was consistent with the results of the Japanese scholar Asikin.29 In NCS, a total of 56 odor components were identified, including the volatile compounds categorized by 10 volatile types; 5 alcohols, 6 aldehydes, 9 ketones, 12 carboxylic acids, 10 pyrazines, 7 phenols, 4 heterocyclic compounds, 1 ester, 3 hydrocarbons, and 1 sulfur-containing compound. Carboxylic acids constituted the largest group of components in NCS (10 775.00 ng g−1), followed by the aldehydes and phenols (8228.72 and 7561.06 ng g−1 respectively), while sulfur compounds constituted the lowest content. Considering the content of each volatile component, the most dominant odor components were 3,5-dimethoxy-4-hydroxybenzaldehyde, hexadecanoic acid, 2,6-di-tert-butyl-p-methylphenol, acetic acid, 4-ethenyl-2-methoxyphenol, 4-hydroxy-3-methoxybenzaldehyde, and 4-hydroxy-2,5-dimethyl-3(2H)furanone. Of these odor compounds, 5-methyl furfuryl alcohol, 3,5-dimethoxy-4-hydroxybenzaldehyde, 2-hydroxy-3-methyl-2-cyclopentene-1-one, 3-hydroxy-4,4-dimethyldihydro-2(3H)-furanone, 4-hydroxy-2,5-dimethyl-3(2H)furanone, 4′-hydroxyacetophenone, and γ-butyrolactone provide a caramel, sweet or cotton candy flavor to the foods, while furfural and pyrazines provide a roasted or cooked flavor to the foods.18,30,31 Besides, (Z)-3-hexen-1-ol, hexanal, (E)-2-nonenal and nonanal exhibit grass and green flavor.18,26,32 They combine with other odor compounds to form the profile of NCS (Table 2).18
Volatile aroma components of the samples from sugarcane to NCSa,b.
| No. | Component | Odor description | Content (ng g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugarcane | Juice extraction | Juice extraction mixture | Juice extraction mixture with calcium oxide and sodium bicarbonate | Clean juice | Impurities | Concentrated juice | Syrup | NCS | |||
| 1 | 3-Methyl-1-butanol | Whiskey, malt, burnt | ND | ND | 134.85 ± 11.21 | ND | 110.71 ± 3.67 | ND | ND | ND | ND |
| 2 | 2-Heptanol | Mushroom | 73.28 ± 4.53 | 354.02 ± 31.04 | 187.1 ± 44.44 | 79.38 ± 4.83 | ND | ND | ND | ND | ND |
| 3 | (Z)-2-Penten-1-ol | Green, plastic, rubber | ND | 99.8 ± 14.6 | 33.12 ± 7.61 | 22.8 ± 7.01 | ND | ND | ND | ND | ND |
| 4 | 1-Hexanol | Resin, flower, green | 64.16 ± 4.53 | 60.96 ± 7.49 | 58.4 ± 17.48 | 387.77 ± 35.45 | 38.84 ± 1.18 | 45.97 ± 3.52 | ND | ND | ND |
| 5 | (E)-3-Hexen-1-ol | Moss, fresh | ND | ND | ND | ND | 8.34 ± 0.36 | ND | ND | ND | ND |
| 6 | (Z)-3-Hexen-1-ol | Grass | 25.32 ± 12.79 | 64.53 ± 10.56 | 15.21 ± 2.75 | 68.91 ± 9.85 | ND | ND | ND | ND | ND |
| 7 | 1-Octen-3-ol | Mushroom | 94.76 ± 25.1 | 111.08 ± 1.23 | 65.94 ± 11.54 | 80.31 ± 5.34 | 22.79 ± 2.16 | ND | ND | ND | ND |
| 8 | 2-Ethyl-1-hexanol | Rose, green | 59.76 ± 4.63 | 82.23 ± 5.82 | 47.6 ± 6.66 | 77.8 ± 8.14 | ND | ND | 15.63 ± 1.52 | ND | 21.17 ± 0.57 |
| 9 | Octan-1-ol | Chemical, metal, burnt | ND | 84.63 ± 18.82 | 35.78 ± 2.3 | ND | ND | ND | ND | ND | ND |
| 10 | 2,3-Butanediol | Fruit, onion | 68.96 ± 11.2 | 159.37 ± 1.4 | 199.56 ± 34.34 | 182.03 ± 15.35 | 68.04 ± 1.68 | 104.99 ± 2.99 | 687.98 ± 24.6 | 347.95 ± 17.41 | 130.25 ± 1.97 |
| 11 | 1,2-Propanediol | Sweet | ND | ND | ND | ND | ND | ND | 93.12 ± 3.39 | ND | ND |
| 12 | Furfuryl alcohol | Burnt | ND | ND | 74.62 ± 11.44 | ND | ND | 138.77 ± 11.54 | 54.89 ± 1.53 | 1473.03 ± 144.45 | 414.87 ± 33.82 |
| 13 | 5-Methyl furfuryl alcohol | Sweet caramellic | ND | ND | ND | ND | ND | ND | ND | 251.87 ± 30.69 | 124.22 ± 0.36 |
| 14 | Phenylethyl alcohol | Honey, spice, rose, lilac | 56.32 ± 13.04 | 166.08 ± 46.4 | 245.62 ± 33.62 | 255.95 ± 18.14 | 104.6 ± 5.08 | 65.91 ± 4.24 | ND | ND | ND |
| 15 | Dodecan-1-ol | Fat, wax | ND | 228.3 ± 30.58 | 183.14 ± 29.36 | 272.82 ± 42.85 | ND | ND | 89.85 ± 4.48 | ND | 129.34 ± 1.97 |
| Content of total alcohols | 442.56 | 1410.99 | 1280.95 | 1427.78 | 353.32 | 355.65 | 941.47 | 2072.84 | 819.85 | ||
| 16 | Hexanal | Grass, tallow, fat | ND | ND | ND | ND | 57.57 ± 2.39 | 133.34 ± 7.09 | 148.22 ± 19.81 | 617.14 ± 16.45 | 186.87 ± 4.67 |
| 17 | (E)-2-Octenal | Green, nut, fat | 20.27 ± 6.51 | 180.94 ± 5.01 | 53.7 ± 22.06 | ND | ND | ND | ND | ND | ND |
| 18 | Nonanal | Fat, citrus, green | 30.94 ± 21.66 | 230.13 ± 16.01 | 43.34 ± 16.63 | 88.91 ± 10.96 | 17.36 ± 0.92 | 36.23 ± 1.08 | 11.08 ± 0.66 | ND | ND |
| 19 | (E,E)-2,4-Heptadienal | Nut, fat | 55.9 ± 14.59 | 71.79 ± 4.45 | 24.92 ± 2.93 | 51.92 ± 6.8 | ND | ND | ND | ND | ND |
| 20 | Furfural | Bread, almond, sweet | ND | ND | ND | ND | ND | ND | 28.04 ± 1.06 | ND | ND |
| 21 | Benzaldehyde | Almond, burnt sugar | 119.65 ± 11.01 | 98.84 ± 10.86 | 79.58 ± 5.07 | 76.26 ± 6.18 | 39.03 ± 1.41 | 50.68 ± 2.49 | 57.94 ± 5.07 | ND | ND |
| 22 | (E)-2-Nonenal | Cucumber, fat, green | ND | 71.11 ± 2.78 | 33.8 ± 2.55 | ND | ND | ND | ND | 137.19 ± 8.92 | 37.81 ± 1.86 |
| 23 | 5-Methylfurfural | Almond, caramel | ND | ND | ND | ND | ND | ND | ND | 77.78 ± 0.19 | 17.53 ± 0.41 |
| 24 | (E,E)-2,4-Decadienal | Fried, wax, fat | 439.41 ± 28.06 | ND | 187.24 ± 46.16 | 326.48 ± 34.11 | ND | 113.13 ± 4.93 | ND | ND | ND |
| 25 | 2-Hydroxymethyl-5-furfural | Cardboard | ND | ND | ND | ND | ND | ND | 115.37 ± 14.96 | 888.06 ± 94.33 | 371.11 ± 18.02 |
| 26 | 4-Hydroxy-3-methoxybenzaldehyde | Vanilla | 319.42 ± 17.54 | ND | ND | ND | 498.56 ± 49.07 | 640.45 ± 23.51 | 898.68 ± 34.91 | 1843.82 ± 157.85 | 1311.55 ± 0.34 |
| 27 | 3,5-Dimethoxy-4-hydroxybenzaldehyde | Sweet cocoa chocolate | ND | ND | ND | ND | 1696.47 ± 142.65 | ND | 3113.45 ± 177.85 | 5846.77 ± 579.38 | 6303.85 ± 3.77 |
| Content of total aldehydes | 985.59 | 652.81 | 422.57 | 543.58 | 2308.99 | 973.83 | 4372.77 | 9410.76 | 8228.72 | ||
| 28 | 2-Methyl-4,5-dihydro-3(2H)-furanone | Nutty, creamy almond | ND | ND | ND | ND | ND | ND | ND | 398.65 ± 17.61 | 74.61 ± 1.45 |
| 29 | 1-Hydroxy-2-propanone | Sweet slightly green burnt | ND | ND | 38.26 ± 3.22 | 17.03 ± 0.46 | 97.36 ± 3.67 | 242.26 ± 10.96 | 258.23 ± 5.82 | 1777.45 ± 104.06 | 516.14 ± 4.19 |
| 30 | 3-Hydroxy-2-butanone | Butter, cream | 417.1 ± 29.88 | 313.21 ± 17.62 | 188.61 ± 11.72 | 79.05 ± 24.09 | 201.81 ± 7.48 | 162.97 ± 11.42 | 74.63 ± 1.72 | 371.32 ± 35.74 | 86.79 ± 0.78 |
| 31 | 1-Hydroxy-2-butanone | brown, oily | ND | ND | ND | ND | ND | ND | ND | 98.5 ± 6.23 | 16.81 ± 1.11 |
| 32 | Acetoxyacetone | Fruity buttery dairy nutty | ND | ND | ND | ND | ND | ND | ND | 354.15 ± 12.62 | 66.27 ± 1.43 |
| 33 | 2-Hydroxy-3-methyl-2-cyclopentene-1-one | Caramellic | ND | ND | ND | ND | 37 ± 3.86 | 39.51 ± 0.71 | 115.81 ± 14.4 | 794.49 ± 51 | 243.33 ± 6.69 |
| 34 | 3-Hydroxy-4,4-dimethyldihydro-2(3H)-furanone | Cotton candy | ND | ND | ND | ND | 20.2 ± 0.75 | ND | 112.72 ± 7.62 | 666.42 ± 1.23 | 266.96 ± 0.59 |
| 35 | Dihydro-5-pentyl-2(3H)-furanone | Coconut, peach | 141.12 ± 10.26 | 98.87 ± 0.42 | 88.02 ± 9.44 | 132.67 ± 28.83 | ND | ND | 138.46 ± 7.91 | ND | ND |
| 36 | 4-Hydroxy-2,5-dimethyl-3(2H)furanone | Caramel | ND | ND | ND | ND | ND | ND | 139.48 ± 9.36 | 1880.31 ± 134.62 | 1203.04 ± 7.57 |
| 37 | 4′-Hydroxyacetophenone | Sweet | ND | ND | ND | ND | 114.48 ± 13.64 | ND | 334.31 ± 38.35 | 1503.68 ± 102.9 | 704.24 ± 51.07 |
| Content of total ketones | 558.22 | 412.08 | 314.89 | 228.75 | 470.84 | 444.74 | 1173.64 | 7844.97 | 3178.19 | ||
| 38 | Acetic acid | Sour | 1376.77 ± 52.9 | 930.55 ± 50.79 | 573.6 ± 177.51 | 341.96 ± 38.33 | 97.36 ± 6.24 | 80.23 ± 3.26 | 2148.92 ± 60.78 | 10 164.08 ± 260.85 | 2463.13 ± 7.38 |
| 39 | Formic acid | Acetic, astringent, fruity | 66.86 ± 2.29 | 112.39 ± 3.9 | 63.64 ± 19.66 | 72.46 ± 4.75 | 7.87 ± 0.47 | 16.67 ± 3.4 | ND | 299.61 ± 3.48 | 56.09 ± 12.52 |
| 40 | Propionic acid | Pungent, rancid, soy | 41.33 ± 26.59 | 165.89 ± 7.18 | 230.22 ± 77.58 | 149.73 ± 16.84 | ND | ND | 179.16 ± 15.47 | 550.42 ± 54.71 | 137.68 ± 0.69 |
| 41 | 2-Methylpropionic acid | Rancid, butter, cheese | 32.15 ± 10.12 | 96.7 ± 26.04 | 113.89 ± 28.44 | ND | ND | ND | 93.88 ± 13.75 | 121.68 ± 8.34 | 24.36 ± 1.01 |
| 42 | Butanoic acid | Rancid, cheese, sweat | 275.45 ± 11.13 | 252.35 ± 22.27 | 2402.51 ± 8.34 | 34.03 ± 1.44 | ND | ND | ND | ND | ND |
| 43 | 3-Methylbutanoic acid | Sweat, acid, rancid | 208.73 ± 3.41 | 108.23 ± 0.6 | 112.45 ± 10.25 | ND | ND | ND | 166.02 ± 10.04 | 262.38 ± 6.85 | 62.38 ± 2.01 |
| 44 | 2-Methylpentanoic acid | Buttery creamy cheesy | ND | ND | ND | ND | ND | ND | 141.3 ± 5.11 | 640.21 ± 63.64 | 167.17 ± 1.89 |
| 45 | Hexanoic acid | Sweat | 432.4 ± 32.32 | 101.4 ± 11.73 | 585.24 ± 59.89 | 609.16 ± 77.02 | 122.5 ± 13.81 | 117.32 ± 0.81 | 536.65 ± 21.68 | 716.4 ± 12.07 | 207.44 ± 1.32 |
| 46 | Octanoic acid | Sweat, cheese | 140.58 ± 14.8 | 124.36 ± 3.26 | 119.19 ± 28 | 141.21 ± 9.64 | ND | ND | 100.98 ± 5.57 | ND | ND |
| 47 | Nonanoic acid | Green, fat | 25.93 ± 2.07 | 163.85 ± 14.34 | 116.54 ± 10.29 | 184.67 ± 26.68 | ND | ND | ND | ND | ND |
| 48 | Benzoic acid | Urine | 294.41 ± 11.66 | 403.53 ± 27.46 | 84.07 ± 5.07 | 269.58 ± 16.36 | 24.93 ± 1.19 | 54.96 ± 1.76 | 504.57 ± 31.16 | 2501.43 ± 253.77 | 830.57 ± 10.77 |
| 49 | Dodecanoic acid | Metal | 244.62 ± 22.28 | 538.28 ± 122.99 | 390.57 ± 92.89 | 641.23 ± 8.33 | 66.46 ± 7.74 | 451.04 ± 34.01 | ND | ND | ND |
| 50 | Phenylacetic acid | Honey, flower | ND | ND | ND | ND | ND | ND | 289.68 ± 25.4 | 1060.23 ± 42.56 | 376.82 ± 1.15 |
| 51 | Tetradecanoic acid | Sweet spicy | 293.71 ± 24.51 | 1090.19 ± 197.69 | 367 ± 13.29 | 574.2 ± 39.73 | 39.44 ± 4.76 | 570.49 ± 79.13 | ND | ND | 131.85 ± 30.78 |
| 52 | Pentadecanoic acid | Waxy | 544.77 ± 72.84 | 1386.5 ± 224.7 | 1714.73 ± 226.34 | 2149.31 ± 131.84 | ND | ND | ND | ND | ND |
| 53 | 3-Phenyl-2-propenoic acid | Balsam sweet storax | ND | ND | ND | ND | ND | 223.11 ± 30.29 | 77.8 ± 7.15 | 655.14 ± 76.44 | 311.68 ± 26.44 |
| 54 | Hexadecanoic acid | Slightly waxy fatty | 18 197.82 ± 1624.58 | 25 513 ± 5663.46 | 15 909.23 ± 1186.68 | 36 757.16 ± 3478.92 | 2174.51 ± 222.59 | 24 000.4 ± 4476.98 | 507.96 ± 35.84 | 9670.02 ± 374.61 | 6005.83 ± 1007.13 |
| Content of total carboxylic acids | 22 175.53 | 30 987.23 | 22 782.88 | 41 924.69 | 2533.08 | 25 514.21 | 4746.93 | 26 641.59 | 10 775 | ||
| 55 | 2-Methylpyrazine | Popcorn | ND | ND | 40.81 ± 9.89 | ND | 60.95 ± 1.41 | 110.34 ± 4.34 | ND | 2286.51 ± 134.92 | 490.9 ± 4.5 |
| 56 | 2,6-Dimethylpyrazine | Nut, cocoa, roast beef | ND | ND | ND | ND | 62.73 ± 1.79 | 276.55 ± 12.99 | 55.47 ± 12.78 | 2144.53 ± 63.11 | 508.52 ± 2.03 |
| 57 | 2,5-Dimethylpyrazine | Cocoa, nut, roast beef | ND | ND | ND | ND | 124.13 ± 2.91 | 495.53 ± 27.76 | 200.17 ± 16.26 | 3661.12 ± 84.37 | 876.89 ± 11.19 |
| 58 | 2,3-Dimethylpyrazine | Nut, butter, cocoa | ND | ND | ND | ND | ND | ND | ND | 557.09 ± 22.08 | 127.01 ± 1.49 |
| 59 | 2-Ethyl-5-methylpyrazine | Fruit, sweet | ND | ND | ND | ND | ND | ND | ND | 330.18 ± 1.83 | 78.85 ± 1.11 |
| 60 | 2,3,5-Trimethylpyrazine | Roast, potato, must | ND | ND | ND | ND | 7.85 ± 0.25 | 24.83 ± 0.41 | ND | ND | 155.97 ± 5.77 |
| 61 | 2-Ethyl-3-methylpyrazine | Roast | ND | ND | ND | ND | ND | ND | ND | 243.51 ± 37.25 | ND |
| 62 | 2-Ethyl-6-methylpyrazine | Roasted hazelnut | ND | ND | ND | ND | ND | ND | ND | 288.59 ± 22.5 | 44.01 ± 7.33 |
| 63 | 3-Ethyl-2,5-dimethylpyrazine | Potato, roast | ND | ND | ND | ND | ND | ND | ND | 497.77 ± 17.81 | 159.13 ± 0.22 |
| 64 | 2-Methyl-6-vinylpyrazine | Hazelnut | ND | ND | ND | ND | ND | ND | ND | 325 ± 4.84 | 176.47 ± 29.72 |
| 65 | 2-Acetyl-6-methylpyrazine | Roasted cocoa popcorn | ND | ND | ND | ND | ND | ND | ND | 202.06 ± 17.07 | 75.28 ± 1.08 |
| Content of total pyrazines | 0 | 0 | 40.81 | 0 | 255.66 | 907.26 | 255.63 | 10 536.36 | 2693.02 | ||
| 66 | 2,6-Di-tert-butyl-p-methylphenol | Mild phenolic camphor | 5478.99 ± 266.6 | 3584.08 ± 45.89 | 4556.2 ± 288.51 | 7389.02 ± 181.61 | 3669.82 ± 226.64 | 4267.69 ± 375.35 | 5051.27 ± 204.36 | 16 619.31 ± 1567.29 | 4703.82 ± 34.11 |
| 67 | 4-Allyl-2-methoxyphenol | Clove, honey | 59.95 ± 7.05 | 92.64 ± 6.08 | 67.88 ± 1.96 | 88.87 ± 9.84 | ND | ND | ND | ND | ND |
| 68 | 4-Ethenyl-2-methoxyphenol | Clove, curry | 390.62 ± 64.25 | 348.34 ± 32.47 | 742.18 ± 63.35 | ND | 61.32 ± 6.63 | 368.69 ± 18.49 | 531.51 ± 36.27 | 3150.75 ± 115.73 | 1471.55 ± 1.65 |
| 69 | 2,4-Di-tert-butylphenol | Phenolic | 353.56 ± 18.06 | 469.01 ± 46.97 | 378.14 ± 31.39 | 543.08 ± 37.15 | 214.62 ± 12.87 | 232.15 ± 11.5 | 224.57 ± 6.91 | 707.51 ± 40.33 | 268.09 ± 3.04 |
| 70 | 4-Ethenyl-2,6-dimethoxy-phenol | Phenolic animal leather | ND | ND | ND | ND | ND | 70.06 ± 12.03 | 62.38 ± 8.91 | 855.05 ± 50.29 | 488.31 ± 1.16 |
| 71 | 4-Allyl-2,6-dimethoxyphenol | Sweet, flower | ND | ND | ND | ND | ND | ND | 44.84 ± 7.27 | ND | 276.65 ± 14.93 |
| 72 | 2-Methoxy-4-acetylphenol | Vanilla | ND | ND | ND | ND | 44.5 ± 3.61 | 71.95 ± 11.5 | 169.93 ± 2.36 | 401.52 ± 32.08 | 352.64 ± 29.38 |
| Content of total phenols | 6283.11 | 4494.07 | 5744.4 | 8020.97 | 3990.27 | 5010.54 | 6084.5 | 21 734.13 | 7561.06 | ||
| 73 | 2-Pentylfuran | Green bean, butter | 21.94 ± 2.37 | 38.56 ± 9.98 | ND | ND | ND | 121.82 ± 6.95 | 57.29 ± 4.18 | ND | ND |
| 74 | 2-Acetylfuran | Balsamic | ND | ND | ND | ND | ND | ND | ND | 238.34 ± 2 | 38.42 ± 2.34 |
| 75 | 2-Acetylpyrrole | Nut, walnut, bread | ND | ND | ND | ND | ND | ND | ND | 1706.52 ± 45.79 | 549.07 ± 0.08 |
| 76 | 2-Formylpyrrole | Musty beefy coffee | ND | ND | ND | ND | ND | ND | ND | 156.96 ± 17.32 | 45.15 ± 0.69 |
| Content of total heterocyclic compounds | 21.94 | 38.56 | 0 | 0 | 0 | 121.82 | 57.29 | 2101.82 | 632.64 | ||
| 77 | γ-Butyrolactone | Caramel, sweet | ND | ND | ND | ND | ND | ND | ND | 268.1 ± 12.08 | ND |
| 78 | Ethyl hexadecanoate | Wax | ND | 276.06 ± 24.82 | 481.06 ± 9.63 | ND | ND | ND | ND | ND | ND |
| 79 | Dibutyl phthalate | Faint odor | 842.87 ± 58.86 | 1851.36 ± 19.74 | 1219.2 ± 237.32 | 1193.5 ± 101.33 | 609.02 ± 57.37 | 472.54 ± 80.95 | 292.49 ± 4.87 | 2048.77 ± 110.4 | 669.07 ± 17.22 |
| Content of total esters | 842.87 | 2127.42 | 1700.26 | 1193.5 | 609.02 | 472.54 | 292.49 | 2316.88 | 669.07 | ||
| 80 | 1,3-Dimethylbenzene | Plastic | 282.8 ± 25.34 | 203.27 ± 12.47 | 292.97 ± 2.74 | 145.13 ± 11.63 | 323.75 ± 4.53 | 264.48 ± 15.21 | 262.02 ± 18.66 | 582.61 ± 57.56 | 229.84 ± 5.76 |
| 81 | (+)-Limonene | Citrus, mint | ND | 156.75 ± 19.72 | 52.15 ± 6.7 | 177.35 ± 14.24 | ND | 192.81 ± 10.62 | 175.02 ± 9.54 | ND | 335.84 ± 0.99 |
| 82 | Styrene | Balsamic, gasoline | 369.72 ± 17.77 | 290.29 ± 30.56 | 363.78 ± 29.47 | 338.22 ± 35.36 | 333.47 ± 11.19 | 273.88 ± 21.24 | 284.12 ± 4 | 678.75 ± 58.86 | 254.61 ± 3.7 |
| Content of total hydrocarbons | 652.52 | 650.31 | 708.9 | 660.7 | 657.22 | 731.16 | 721.16 | 1261.36 | 820.29 | ||
| 83 | Dimethyl sulfoxide | Garlic | ND | ND | ND | ND | 23.54 ± 0.91 | 135.25 ± 4.24 | ND | 343.01 ± 2.12 | 68.4 ± 2.69 |
| 84 | Methyl sulfone | Sulfur, burnt | ND | ND | ND | ND | 96.99 ± 7.09 | 69.53 ± 8.03 | 80.49 ± 11.46 | 74.75 ± 0.49 | ND |
| Content of total sulfur compounds | 0 | 0 | 0 | 0 | 120.54 | 204.78 | 80.49 | 417.76 | 68.4 | ||
| Total identified/detected | 31 962.36 | 40 773.46 | 32 995.66 | 53 999.97 | 11 298.94 | 34 736.52 | 18 726.37 | 84 338.47 | 35 446.24 | ||
ND: not detected.
Content stated as the mean ± SD (n = 3) and the unit was nanogram per gram sample.
3.2. Aroma extraction dilution analysis (AEDA) and the generation of several compounds with high FD factor
The AEDA was employed to characterize the contribution of key aroma compounds in the NCS. A total of 20 compounds, including 5 ketones, 2 aldehydes, 2 phenols, 1 alcohol, 4 pyrazines, 5 carboxylic acids, and 1 hydrocarbon were detected with FD factors of ≥ 1 (Table 3). It can be seen that 4-hydroxy-2,5-dimethyl-3(2H)furanone has the largest FD factor (FD = 2187) and provides a strong caramel odor. According to the current research, 4-hydroxy-2,5-dimethyl-3(2H)furanone can be biosynthesized in strawberry using radiotracer33 and cyclization of the intact skeleton.34 In contrast, for sugarcane, juice extraction, and clean juice have no 4-hydroxy-2,5-dimethyl-3(2H)furanone was detected. It is most likely formed by the Maillard reaction through deoxy sugars.33 Followed by (E)-2-nonenal, 2-hydroxy-3-methyl-2-cyclopentene-1-one, and 4-allyl-2,6-dimethoxyphenol with a FD of 729, showing cucumber, caramellic and flower odor respectively. (E)-2-nonenal is an unsaturated aldehyde with a cucumber and grass odor generated during the peroxidation of fatty acids.35–37 However, oxygen will intensify (E)-2-nonenal degradation to nonenoic acid.38 The (E)-2-nonenal in the juice extraction and juice extraction mixture might have resulted due to the microbial and enzymatic effects.39 However, the longer time contact with oxygen may oxidize the (E)-2-nonenal to nonenoic acid.38 Therefore, the (E)-2-nonenal in the clean juice and concentrated juice was not detectable. The FD factor of 2-hydroxy-3-methyl-2-cyclopentene-1-one is also 729 but was not detected in the previous steps until the clean juice. This might attribute to the formatting of the Maillard reaction products during relatively high temperatures.40 Due to the strong caramel like odor, 2-hydroxy-3-methyl-2-cyclopentene-1-one is always organoleptically classified in the same category as the furanones and pyranones and is often used as a flavoring agent in coffee and caramel. This compound was also identified as a key aroma compound of roasted coffee.41 The last compound of the FD factor of 729 was 4-allyl-2,6-dimethoxyphenol, which exhibited a strong antioxidant activity with a sweet and flower odor, that was identified as the key aroma components of wine and crude drugs.42,43 However, the formation of 4-allyl-2,6-dimethoxyphenol is not clear to date. 4-Ethenyl-2-methoxyphenol exhibited a clove and curry like odor and its FD factor was 243. To date, a few studies on its generation have been reported. One of the reports demonstrated that it originates probably from glycosidic bound 2-methoxy-4-vinylphenol.44 It is well known that pyrazines are present in a wide variety of heated foodstuffs, notably in the fried or roasted foods, such as potato chips, coffee, cocoa, popcorn, roasted peanuts.45 De Kimpe studied the formation of flavour compounds from the model reactions of 20 amino acids with ascorbic acid and thirty-six different pyrazines were identified, mostly ethyl and methyl substituted pyrazines.46 Besides amino acids, oligopeptides from hydrolyzed whey protein can also contribute significantly to an increased amount of pyrazines.47 3-Ethyl-2,5-dimethylpyrazine, a typical Maillard reaction product that contributes a roast flavor to the potato,48 is a key odor compound of NCS with a FD factor of 81. It was first found in the syrup of the 9 samples. At this stage, the content and variety of pyrazines increased greatly. It is speculated that it is related to Maillard reaction occurred due to a prolonged period of high temperature. Hexanoic acid has an FD factor of 81, which exhibits a sweaty odor. It can be detected from the first step to the last step during the NCS production. The initial hexanoic acid comes from sugarcane itself, but in the sugarcane juice, it might be contributed by the microorganisms but the content fluctuates slightly.49 Hexanoic acid content of clean juice and impurities is lower than that of the mixed juice due to the flocculation of polyacrylamide. With evaporation and concentration, hexanoic acid content rises, and then the content decreases due to the volatilization during the drying process. 3-Hydroxy-4,4-dimethyldihydro-2(3H)-furanone was detected neither in the sugarcane nor in the sugarcane juice. It was first detected in the clean juice. The content of 3-hydroxy-4,4-dimethyldihydro-2(3H)-furanone increased in the concentrated juice. It is speculated that it may be related to the thermal reactions, but the specific reason behind the formation is still unclear. This component has also been found in Thai soy sauce.50 The change in the initial content of acetic acid might have happened due to the change in the solid content and microbial effect,51 but the drastic change in the syrup might have happened due to the α-dicarbonyl and β-dicarbonyl cleavage of hexose or pentose during the thermal processing.52 A large number of Bacillus sp. was found in the soil. The content of 2-methylpropionic acid increased in the initial steps due to the microbial fermentation.53–56 The Maillard reaction can produce lower fatty acids. The temperature of the concentration stage was very high, and the small molecular acids in this stage were likely to be produced by the Maillard reactions (Fig. 4).25
Key odor compounds in NCS analyzed by AEDA and their contents in various samplesa,b.
| No. | RI | Component | Odor description | Content (ng g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugarcane | Juice extraction | Juice extraction mixture | Juice extraction mixture with calcium oxide and sodium bicarbonate | Clean juice | Impurities | Concentrated juice | Syrup | NCS | FD factor | ||||
| 1 | 1987 | 4-Hydroxy-2,5-dimethyl-3(2H)furanone | Caramel | ND | ND | ND | ND | ND | ND | 139.48 ± 9.36 | 1880.31 ± 134.62 | 1203.04 ± 7.57 | 2187 |
| 2 | 1491 | (E)-2-Nonenal | Cucumber, fat, green | ND | 71.11 ± 2.78 | 33.8 ± 2.55 | ND | ND | ND | ND | 137.19 ± 8.92 | 37.81 ± 1.86 | 729 |
| 3 | 1784 | 2-Hydroxy-3-methyl-2-cyclopentene-1-one | Caramellic | ND | ND | ND | ND | 37 ± 3.86 | 39.51 ± 0.71 | 115.81 ± 14.4 | 794.49 ± 51 | 243.33 ± 6.69 | 729 |
| 4 | 2476 | 4-Allyl-2,6-dimethoxyphenol | Sweet, flower | ND | ND | ND | ND | ND | ND | 44.84 ± 7.27 | ND | 276.65 ± 14.93 | 729 |
| 5 | 2138 | 4-Ethenyl-2-methoxyphenol | Clove, curry | 390.62 ± 64.25 | 348.34 ± 32.47 | 742.18 ± 63.35 | ND | 61.32 ± 6.63 | 368.69 ± 18.49 | 531.51 ± 36.27 | 3150.75 ± 115.73 | 1471.55 ± 1.65 | 243 |
| 6 | 1428 | 3-Ethyl-2,5-dimethylpyrazine | Potato, roast | ND | ND | ND | ND | ND | ND | ND | 497.77 ± 17.81 | 159.13 ± 0.22 | 81 |
| 7 | 1801 | Hexanoic acid | Sweat | 432.4 ± 32.32 | 101.4 ± 11.73 | 585.24 ± 59.89 | 609.16 ± 77.02 | 122.5 ± 13.81 | 117.32 ± 0.81 | 536.65 ± 21.68 | 716.4 ± 12.07 | 207.44 ± 1.32 | 81 |
| 8 | 1977 | 3-Hydroxy-4,4-dimethyldihydro-2(3H)-furanone | Cotton candy | ND | ND | ND | ND | 20.2 ± 0.75 | ND | 112.72 ± 7.62 | 666.42 ± 1.23 | 266.96 ± 0.59 | 81 |
| 9 | 1404 | Acetic acid | Sour | 1376.77 ± 52.9 | 930.55 ± 50.79 | 573.6 ± 177.51 | 341.96 ± 38.33 | 97.36 ± 6.24 | 80.23 ± 3.26 | 2148.92 ± 60.78 | 10 164.08 ± 260.85 | 2463.13 ± 7.38 | 27 |
| 10 | 1535 | 2-Methylpropionic acid | Rancid, butter, cheese | 32.15 ± 10.12 | 96.7 ± 26.04 | 113.89 ± 28.44 | ND | ND | ND | 93.88 ± 13.75 | 121.68 ± 8.34 | 24.36 ± 1.01 | 27 |
| 11 | 1118 | 1,3-Dimethylbenzene | Plastic | 282.8 ± 25.34 | 203.27 ± 12.47 | 292.97 ± 2.74 | 145.13 ± 11.63 | 323.75 ± 4.53 | 264.48 ± 15.21 | 262.02 ± 18.66 | 582.61 ± 57.56 | 229.84 ± 5.76 | 9 |
| 12 | 1387 | 2,3,5-Trimethylpyrazine | Roast, potato, must | ND | ND | ND | ND | 7.85 ± 0.25 | 24.83 ± 0.41 | ND | ND | 155.97 ± 5.77 | 9 |
| 13 | 1705 | 2-Methylpentanoic acid | Buttery creamy cheesy | ND | ND | ND | ND | ND | ND | 141.3 ± 5.11 | 640.21 ± 63.64 | 167.17 ± 1.89 | 9 |
| 14 | 1064 | Hexanal | Grass, tallow, fat | ND | ND | ND | ND | 57.57 ± 2.39 | 133.34 ± 7.09 | 148.22 ± 19.81 | 617.14 ± 16.45 | 186.87 ± 4.67 | 3 |
| 15 | 1237 | 2-Methyl-4,5-dihydro-3(2H)-furanone | Nutty, creamy almond | ND | ND | ND | ND | ND | ND | ND | 398.65 ± 17.61 | 74.61 ± 1.45 | 3 |
| 16 | 1372 | 2-Ethyl-5-methylpyrazine | Fruit, sweet | ND | ND | ND | ND | ND | ND | ND | 330.18 ± 1.83 | 78.85 ± 1.11 | 3 |
| 17 | 1309 | 2,6-Dimethylpyrazine | Roasted nut, cocoa, beef | ND | ND | ND | ND | 62.73 ± 1.79 | 276.55 ± 12.99 | 55.47 ± 12.78 | 2144.53 ± 63.11 | 508.52 ± 2.03 | 1 |
| 18 | 1342 | 1-Hydroxy-2-butanone | brown, oily, alcoholic | ND | ND | ND | ND | ND | ND | ND | 98.5 ± 6.23 | 16.81 ± 1.11 | 1 |
| 19 | 1683 | 5-Methyl furfuryl alcohol | Sweet caramellic | ND | ND | ND | ND | ND | ND | ND | 251.87 ± 30.69 | 124.22 ± 0.36 | 1 |
| 20 | 2777 | 3-Phenyl-2-propenoic acid | Balsam sweet storax | ND | ND | ND | ND | ND | 223.11 ± 30.29 | 77.8 ± 7.15 | 655.14 ± 76.44 | 311.68 ± 26.44 | 1 |
ND: not detected.
Content stated as the mean ± SD (n = 3) and the unit was nanogram per gram sample.
Fig. 4. The 10 compounds content with the largest FD factor in different processing stages.
3.3. Principal component analysis (PCA) of different processing stages
The principal component analysis of all the odor compounds of 9 samples from different stages was carried out using the SIMCA 14.1 software (Fig. 5). As illustrated in Fig. 5, the four samples of sugarcane, juice extraction, juice extraction mixture, and juice extraction mixture with calcium oxide and sodium bicarbonate fall in the same area, indicating that the samples in these four stages did not change much. Moreover, no thermal reaction was observed until this step. Therefore, the odor components were close to each other in these 4 samples. Slight changes in the acid and phenol compounds were also observed, but no significant changes were observed for other types of compounds. Perhaps this is the reason, these compounds fall in the same area. Butanoic acid and 3-methyl-1-butanol also fall in the same area with these 4 samples, indicating that the two components exhibit a strong effect on the original sample odor.
Fig. 5. PCA analysis of different processing stages.
In the clarification stage, the temperature increased, and the thermal reaction occurred, which made it different from the first four stages. The clean juice, impurities, and concentrated juice were grouped into one category, (+)-limonene, (E)-3-hexen-1-ol, and 1,2-propanediol fell in the same area, which proved that these three compounds contributed significantly to the odor of these 3 samples.
The syrup falls in an area alone, which proves that it is significantly different from other samples. In fact, the temperature and heating time required to form the syrup were much higher and longer than the other stages, followed by the occurrence of the complex chemical reactions, such as Maillard reactions. 3-Ethyl-2,5-dimethylpyrazine, 2-acetylpyrrole, 2-formylpyrrole, furfuryl alcohol, 2-acetyl-6-methylpyrazine, 2-methylpyrazine, 2-acetyl-5-methylpyrazine, and 2,3-dimethylpyrazine fall in the same area with syrup. These compounds are the products of the Maillard reaction, making the syrup completely different from other samples. Therefore, the Maillard reaction plays a vital role in differentiating the syrup from other samples.
The NCS also aggregated into a separate group. 2,3-Butanediol, 4-allyl-2,6-dimethoxyphenol, and 2,3,5-trimethylpyrazine fell into this area. However, pyrazines originally fell in the syrup area and did not appear in the NCS area again. This happened due to the loss of most of the odorants during the drying process, resulting in reduced odor intensity and complexity. Therefore, the final drying process is critical and should be observed carefully to obtain the NCS with a strong odor. If the drying time and the contact area with the air can be reduced, then better complexity and concentration of the NCS can be obtained.
3.4. Heatmap analysis of different processing stages
To compare the differences among the odor compound contents of the 9 samples, the NCS odor analysis results were plotted into a heatmap, as depicted in Fig. 6. Each column in the figure represents samples from different production steps, and each row represents a different kind of odor compound. The different colors in the figure indicate the level of content, such as white refers to the compounds with average content; green refers to the compounds with lower content, and; red refers to the compounds with higher content.
Fig. 6. Heatmap analysis of different processing stages.
It can be observed from the heatmap that the first 4 stages exhibited similarity and can be divided into a group. The compounds with red color might be the endogenous components of plants. These compounds are mainly long-chain alcohols and carboxylic acids.57,58 Among them, phenylethyl alcohol and (Z)-3-hexen-1-ol have been proved to be one of the odor compounds of sugarcane juice.59,60 Compounds, such as butanoic acids, are one of the products of microbial action.59,60
The clean juice, impurity, and concentrated juice can be classified into another group. The compounds with high contents in the three samples showed green color in the first four stages, which proved that the content was distinctly reduced. Moreover, only a few compounds in these three stages show red color, which proved that the content of odor compounds was overall low. This phenomenon may attribute to the loss of odor compounds during the clarification process, which reduces the content of odor compounds.
Syrup samples can be divided into a single group. The Maillard reaction products such as pyrazines and furanones primarily cluster together in this area. It is speculated that the Maillard reaction occurring at high temperature at this stage makes syrup obviously different from other samples. The sample in the last column was the NCS, and the color was lighter than the last column. However, the light-colored areas and dark-colored areas in the 2 samples were similar, which might be caused by the loss of odor compounds during the drying process.
4. Conclusion
In conclusion, a total of 84 odor compounds extracted from the 9 samples from different stages in the NCS production line through a liquid–liquid extraction method were detected by GC-O-MS. Further, the AEDA technique revealed 20 key aroma compounds of NCS. Of them, 4-hydroxy-2,5-dimethyl-3(2H)furanone (caramel) exhibited the highest FD factor of 2187, followed by (E)-2-nonenal (cucumber), 2-hydroxy-3-methyl-2-cyclopentene-1-one (caramel), 4-allyl-2,6-dimethoxyphenol (sweet, flower), 4-ethenyl-2-methoxyphenol (clove), 3-ethyl-2,5-dimethylpyrazine (potato, roast), hexanoic acid (sweaty), 3-hydroxy-4,4-dimethyldihydro-2(3H)-furanone (cotton candy), acetic acid (vinegar), and 2-methylpropionic acid (rancid, cheese) having FD factors from 27 to 729 in NCS.
In the production line, the syrup stage is considered as the key step in the formation of NCS odor. During this stage, the Maillard reaction generates a variety of Maillard reaction products, such as pyrazines, pyrroles, and furans, which adds complexity and intensity to NCS odor. However, the final cooling and drying stage causes a severe loss of odor compounds. Therefore, we concluded that shortening the cooling time or reducing the contact area of NCS with air could enrich the odor of the NCS.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
Authors greatly acknowledge the panelists of Laboratory of Molecular Sensory Science, Beijing Technology and Business University.
References
- Moore P. H. and Botha F. C., Sugarcane: physiology, biochemistry and functional biology, John Wiley & Sons, 2013 [Google Scholar]
- Sindhu R. Gnansounou E. Binod P. Pandey A. Renewable Energy. 2016;98:203–215. doi: 10.1016/j.renene.2016.02.057. [DOI] [Google Scholar]
- Li Y. R. Yang L. T. Sugar Tech. 2015;17:1–8. doi: 10.1007/s12355-014-0342-1. [DOI] [Google Scholar]
- Kumbhar Y. S. Int. J. Res. Eng. Technol. 2016;3:590–594. [Google Scholar]
- Velasquez F. Espitia J. Mendieta O. Escobar S. Rodriguez J. J. Food Eng. 2019;255:32–40. doi: 10.1016/j.jfoodeng.2019.03.009. [DOI] [Google Scholar]
- Levine R. A. Smith R. E. J. Agric. Food Chem. 2005;53:4410–4416. doi: 10.1021/jf047887t. [DOI] [PubMed] [Google Scholar]
- Cook D. J. Taylor A. J. J. Agric. Food Chem. 2005;53:8926–8933. doi: 10.1021/jf051585p. [DOI] [PubMed] [Google Scholar]
- Li M. X. Li M. L. Li Q. T. Dai Y. C. Wang Z. L. China Condiment. 2019;44:182–186. [Google Scholar]
- Fu X. Zhou H. F. Li C. Zhao Z. G. Yu S. J. Mod. Food Sci. Technol. 2014:17–22. [Google Scholar]
- Scalbert A. Johnson I. T. Saltmarsh M. Am. J. Clin. Nutr. 2005;81:215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- Korkina L. Cell. Mol. Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- Xu L. J. Yuan Y. M. Feng A. G. Deng J. Hu X. P. Food Sci. 2018;39:125–130. [Google Scholar]
- Lee J. S. Ramalingam S. Jo I. G. Kwon Y. S. Bahuguna A. Oh Y. S. Kwon O. J. Kim M. Food Res. Int. 2018;109:614–625. doi: 10.1016/j.foodres.2018.04.047. [DOI] [PubMed] [Google Scholar]
- Feng S. Luo Z. Zhang Y. Zhong Z. Lu B. Food Chem. 2014;151:452–458. doi: 10.1016/j.foodchem.2013.11.057. [DOI] [PubMed] [Google Scholar]
- Nguyen D. M. T. Doherty W. O. S. Int. Sugar J. 2012;114:309–315. [Google Scholar]
- Xu L. J. Yuan Y. M. Hu X. P. Food Res. Dev. 2017;38:209–214. [Google Scholar]
- EFSA Panel on Dietetic Products and Allergies N. EFSA J. 2010;8:1489. [Google Scholar]
- Asikin Y. Kamiya A. Mizu M. Takara K. Tamaki H. Wada K. Food Chem. 2014;149:170–177. doi: 10.1016/j.foodchem.2013.10.089. [DOI] [PubMed] [Google Scholar]
- Huang S. R. Hang F. X. Wei C. B. Xie C. F. Li K. China Condiment. 2019;44:146–151. [Google Scholar]
- Juliana M. G. Paulo C. N. Francisco J. H. Orjuela A. Osorio C. Food Chem. 2017;228:7–13. doi: 10.1016/j.foodchem.2017.01.134. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Ishmael M. Asikin Y. Hirose N. Mizu M. Shikanai T. Tamaki H. Wada K. J. Food Sci. 2016;81:C2647–C2655. doi: 10.1111/1750-3841.13531. [DOI] [PubMed] [Google Scholar]
- Xi Y. R. Tang K. Xu Y. Wang D. Wang Q. W. Zhang H. N. Food and Fermentation Industries. 2016:192–197. [Google Scholar]
- Forero D. P. Orrego C. E. Peterson D. G. Osorio C. Food Chem. 2015;169:85–91. doi: 10.1016/j.foodchem.2014.07.111. [DOI] [PubMed] [Google Scholar]
- Chen S. Wang C. C. Qian M. Li Z. Xu Y. J. Agric. Food Chem. 2019;67:4876–4884. doi: 10.1021/acs.jafc.9b01420. [DOI] [PubMed] [Google Scholar]
- Martins S. I. Jongen W. M. Van Boekel M. A. Trends Food Sci. Technol. 2000;11:364–373. doi: 10.1016/S0924-2244(01)00022-X. [DOI] [Google Scholar]
- Asikin Y. Takahara W. Takahashi M. Hirose N. Ito S. Wada K. Food Anal. Methods. 2017;10:1844–1856. doi: 10.1007/s12161-016-0746-5. [DOI] [Google Scholar]
- Berlowska J. Cieciura W. Borowski S. Dudkiewicz M. Binczarski M. Witonska I. Otlewska A. Kregiel D. Molecules. 2016;21:14. doi: 10.3390/molecules21101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto T., in Thermal Generation of Aromas, American Chemical Society, 1989, ch. 13, vol. 409, pp. 134–142 [Google Scholar]
- Asikin Y. Hirose N. Tamaki H. Ito S. Oku H. Wada K. LWT--Food Sci. Technol. 2016;66:340–347. doi: 10.1016/j.lwt.2015.10.039. [DOI] [Google Scholar]
- Kaneko S. Sakai R. Kumazawa K. Usuki M. Nishimura O. Biosci., Biotechnol., Biochem. 2013:130112. doi: 10.1271/bbb.130112. [DOI] [PubMed] [Google Scholar]
- Pollnitz A. P. Pardon K. H. Sefton M. A. J. Chromatogr. A. 2000;874:101–109. doi: 10.1016/S0021-9673(00)00086-8. [DOI] [PubMed] [Google Scholar]
- Weerawatanakorn M. Asikin Y. Takahashi M. Tamaki H. Wada K. Ho C. T. Chuekittisak R. J. Food Sci. Technol. 2016;53:4084–4092. doi: 10.1007/s13197-016-2415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab W. Molecules. 2013;18:6936–6951. doi: 10.3390/molecules18066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poisson L. Schaerer A. Spreng S. Mestdagh F. Blank I. Davidek T. J. Agric. Food Chem. 2019;67:13829–13839. doi: 10.1021/acs.jafc.9b00770. [DOI] [PubMed] [Google Scholar]
- Ishino K. Wakita C. Shibata T. Toyokuni S. Machida S. Matsuda S. Matsuda T. Uchida K. J. Biol. Chem. 2010;285:15302–15313. doi: 10.1074/jbc.M109.068023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda Z. Mikulikova R. Belakova S. Benesova K. Marova I. Nesvadba Z. Kvasny Prum. 2010;56:428–432. doi: 10.18832/kp2010044. [DOI] [Google Scholar]
- Tressl R. Drawert F. J. Agric. Food Chem. 1973;21:560–565. doi: 10.1021/jf60188a031. [DOI] [Google Scholar]
- Liégeois C. Meurens N. Badot C. Collin S. J. Agric. Food Chem. 2002;50:7634–7638. doi: 10.1021/jf020617v. [DOI] [PubMed] [Google Scholar]
- Vermeulen N. Czerny M. Gänzle M. G. Schieberle P. Vogel R. F. J. Cereal Sci. 2007;45:78–87. doi: 10.1016/j.jcs.2006.07.002. [DOI] [Google Scholar]
- Yu X. Y. Zhao M. Y. Liu F. Zeng S. T. Hu J. LWT--Food Sci. Technol. 2013;53:22–28. doi: 10.1016/j.lwt.2013.01.024. [DOI] [Google Scholar]
- Schenker S. Heinemann C. Huber M. Pompizzi R. Perren R. Escher R. J. Food Sci. 2002;67:60–66. doi: 10.1111/j.1365-2621.2002.tb11359.x. [DOI] [Google Scholar]
- Rodríguez-Bencomo J. J. Cabrera-Valido H. M. Pérez-Trujillo J. P. Cacho J. Eur. Food Res. Technol. 2011;233:413. doi: 10.1007/s00217-011-1529-2. [DOI] [PubMed] [Google Scholar]
- Ogata M. Hoshi M. Shimotohno K. Urano S. Endo T. J. Am. Oil Chem. Soc. 1997;74:557–562. doi: 10.1007/s11746-997-0180-3. [DOI] [Google Scholar]
- Merks I. Svendsen A. B. Planta Med. 1989;55:88–89. doi: 10.1055/s-2006-961837. [DOI] [PubMed] [Google Scholar]
- Pao Shui W. Odell G. V. J. Agric. Food Chem. 1973;21:868–870. doi: 10.1021/jf60189a032. [DOI] [PubMed] [Google Scholar]
- Adams A. De Kimpe N. Food Chem. 2009;115:1417–1423. doi: 10.1016/j.foodchem.2009.01.071. [DOI] [Google Scholar]
- Scalone G. L. L. Cucu T. De Kimpe N. De Meulenaer B. J. Agric. Food Chem. 2015;63:5364–5372. doi: 10.1021/acs.jafc.5b01129. [DOI] [PubMed] [Google Scholar]
- Reineccius G. A. Andersen D. A. Kavanagh T. E. Keeney P. G. J. Agric. Food Chem. 1972;20:199–202. doi: 10.1021/jf60180a033. [DOI] [Google Scholar]
- Vesely P. Lusk L. Basarova G. Seabrooks J. Ryder D. J. Agric. Food Chem. 2003;51:6941–6944. doi: 10.1021/jf034410t. [DOI] [PubMed] [Google Scholar]
- Wanakhachornkrai P. Lertsiri S. Food Chem. 2003;83:619–629. doi: 10.1016/S0308-8146(03)00256-5. [DOI] [Google Scholar]
- Godshall M. A. Delucca A. J. J. Agric. Food Chem. 1984;32:390–393. doi: 10.1021/jf00122a051. [DOI] [Google Scholar]
- Yuan H. Sun L. Chen M. Wang J. Food Chem. 2018;239:56–61. doi: 10.1016/j.foodchem.2017.06.079. [DOI] [PubMed] [Google Scholar]
- Rodriguez Campos J. Escalona Buendía H. B. Contreras Ramos S. M. Orozco Avila I. Jaramillo Flores E. Lugo Cervantes E. Food Chem. 2012;132:277–288. doi: 10.1016/j.foodchem.2011.10.078. [DOI] [PubMed] [Google Scholar]
- Jollivet N. Bézenger M. C. Vayssier Y. Belin J. M. Appl. Microbiol. Biotechnol. 1992;36:790–794. doi: 10.1007/BF00172196. [DOI] [Google Scholar]
- Rajini K. S. Aparna P. Sasikala C. Ramana C. V. Crit. Rev. Microbiol. 2011;37:99–112. doi: 10.3109/1040841X.2010.512267. [DOI] [PubMed] [Google Scholar]
- Zhu B. F. Xu Y. J. Ind. Microbiol. Biotechnol. 2010;37:815–821. doi: 10.1007/s10295-010-0726-5. [DOI] [PubMed] [Google Scholar]
- Beaulieu J. C. Lea J. M. J. Agric. Food Chem. 2006;54:7789–7793. doi: 10.1021/jf060663l. [DOI] [PubMed] [Google Scholar]
- Liu Y. He C. C. Song H. L. Food Anal. Methods. 2018;11:1677–1689. doi: 10.1007/s12161-018-1153-x. [DOI] [Google Scholar]
- Godshall M., in Sugar Series, Elsevier, 1988, vol. 9, pp. 236–252 [Google Scholar]
- Fadel H. H. M. Mahmoud M. G. Asker M. M. S. Lotfy S. N. Electron. J. Biotechnol. 2015;18:5–9. doi: 10.1016/j.ejbt.2014.10.006. [DOI] [Google Scholar]