Electrochemical and thermodynamic properties of 2-(diethylamino)-4-(1-ethylpropyl)-6-phenyl-benzene-1,3-dicarbonitrile derivatives in the photo-reduction mechanism.
| Acronym | E red vs. Ag/AgCl [V] | E S1 [eV] | ΔGet(S1)a [eV] | E T1 [eV] | ΔGet(T1)a [eV] |
|---|---|---|---|---|---|
| BI-PH | −1.854 | 3.02 | −0.09 | 2.63 | 0.28 |
| BI-PH-CH3 | −1.840 | 3.04 | −0.13 | 2.62 | 0.28 |
| BI-PH-O-CH3 | −1.927 | 3.05 | −0.05 | 2.60 | 0.39 |
| BI-PH-CN | −1.515 | 2.82 | −0.23 | 2.51 | 0.07 |
| BI-PH-SO2-CH3 | −1.604 | 2.89 | −0.21 | 2.63 | 0.03 |
| BI-PH-S-CH3 | −1.771 | 3.02 | −0.18 | 2.60 | 0.23 |
| BI-PH-CF3 | −1.997 | 2.94 | 0.13 | 2.55 | 0.50 |
| BI-1-NPH | −1.813 | 3.08 | −0.19 | 2.47 | 0.40 |
| BI-2-NPH | −1.720 | 2.99 | −0.20 | 2.56 | 0.22 |
| BI-1-AN | −1.735 | 3.05 | −0.24 | 1.78 | 1.01 |
Calculated from the equation: 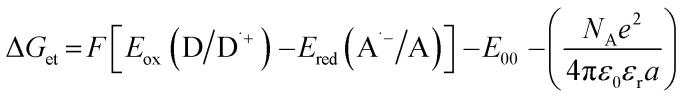 , Eox(D/D˙+) – electrochemically determined oxidation potential of the electron donor (−1.058 V for amine EDB vs. Ag/AgCl)69, Ered (A˙−/A) – electrochemically determined reduction potential of the electron acceptor, ES1 – singlet state energy of the sensitiser determined based on excitation and emission spectra, ET1 – triplet state energy calculated from molecular orbital calculations (uB3LYP/6-31G* level of theory), Φet – quantum yield of electron transfer, Φet = KSV[EDB]/(1 + KSV[EDB]) for the concentration of amine [EDB] = 0.086 mol dm−3.
, Eox(D/D˙+) – electrochemically determined oxidation potential of the electron donor (−1.058 V for amine EDB vs. Ag/AgCl)69, Ered (A˙−/A) – electrochemically determined reduction potential of the electron acceptor, ES1 – singlet state energy of the sensitiser determined based on excitation and emission spectra, ET1 – triplet state energy calculated from molecular orbital calculations (uB3LYP/6-31G* level of theory), Φet – quantum yield of electron transfer, Φet = KSV[EDB]/(1 + KSV[EDB]) for the concentration of amine [EDB] = 0.086 mol dm−3.
