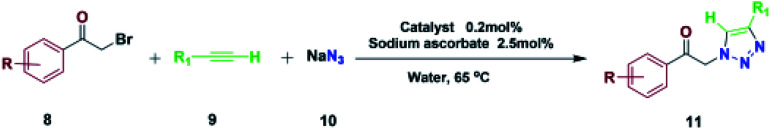
Synthesis of 1,4-disubstituted 1,2,3-triazoles catalyzed by Fe3O4@NFC-ImSalophCua.
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Product | R | R1 | Time (min) | Yieldb (%) | Mp (°C) | TON | Ref. | |
| Found | Reported | ||||||||
| 1 | 11a | Ph | Ph | 30 | 97 | 169–170 | 169–171 | 485 | 46 |
| 2 | 11b | 4-NO2 | Ph | 45 | 90 | 185–186 | 184–186 | 450 | 46 |
| 3 | 11c | 4-Br | Ph | 45 | 95 | 168–169 | 169–170 | 475 | 49 |
| 4 | 11d | Ph | CH2OH | 45 | 90 | 115–116 | 115–117 | 475 | 47 |
| 5 | 11e | 4-NO2 | CH2OH | 60 | 95 | 143–144 | 143–145 | 485 | 49 |
| 6 | 11f | 4-Br | CH2OH | 60 | 85 | 157–158 | 157–159 | 425 | 45 |
| 7 | 11g | Ph | MeCH–OH | 30 | 94 | 128–129 | 127–129 | 470 | 49 |
| 8 | 11h | Ph | Me2C–OH | 45 | 87 | 97–98 | 96–98 | 435 | 48 |
| 9 | 11i | 4-NO2 | Me2C–OH | 90 | 95 | 127–128 | 126–128 | 475 | 49 |
| 10 | 11j | 4-Br | Me2C–OH | 60 | 75 | 169–171 | 170–171 | 375 | 47 |
Reaction conditions: acetophenone (1.0 mmol), alkyne (1.0 mmol), sodium azide (1.2 mmol), catalyst (0.2 mol%) sodium ascorbate (2.5 mol%) and H2O (3 ml), 65 °C.
Isolated yield.
