Abstract
Previous research investigated the cerebral volumetric correlates of impulsivity largely in moderate‐sized samples and few have examined the distinct correlates of dimensions of impulsivity, sex differences, or heritability of the correlates. Here, we performed voxel‐based morphometry analysis of data (n = 11,474; 5,452 girls, 9–10 years) curated from the Adolescent Brain Cognition Development project. In a linear regression with all five UPPS‐P subscores as regressors and age in months, total intracranial volume, study site, and scanner model as covariates, higher levels of lack of premeditation, and sensation seeking were correlated with larger cortical and subcortical gray matter volumes (GMVs). In contrast, higher positive urgency was correlated with smaller GMVs in many of the same regions. The dimensional impulsivity traits also involved distinct volumetric correlates, with, for instance, sensation seeking and positive urgency specifically implicating bilateral caudate head/mid‐cingulate cortex and bilateral lateral orbitofrontal cortex/left precentral gyrus, respectively. Boys relative to girls scored higher in all impulsivity dimensions. Girls relative to boys showed significantly stronger positive and negative correlations between sensation seeking and insula, putamen, and inferior frontal gyrus (IFG) GMVs and between positive urgency and cingulate cortex, insula, and IFG GMVs, respectively. With a subsample of twins, the dimensional impulsivity traits were weakly to moderately heritable in both girls and boys, and the GMV correlates were highly heritable in girls and boys combined. These findings collectively suggest shared and nonshared as well as sex differences in the cerebral volumetric bases of dimensional impulsivity traits and may facilitate research of externalizing psychopathology in children.
Keywords: ABCD, heritability, impulsiveness, UPPS‐P, VBM
Negative urgency, lack of premeditation, lack of perseverance, sensation seeking, and positive urgency involve both shared and nonshared volumetric correlates as well as sex differences in the volumetric correlates. The dimensional impulsivity traits are weakly‐to‐moderately heritable in both girls and boys, and the GMV correlates are highly heritable in girls and boys combined.
1. INTRODUCTION
1.1. Behavioral impulsivity
Impulsivity encompasses a variety of acts “on the spur of the moment” or behaviors without forethoughts (Chamberlain & Sahakian, 2007; Moeller, Barratt, Dougherty, Schmitz, & Swann, 2001). Impulsivity represents a core symptom and behavioral marker of many neuropsychiatric conditions (American Psychiatric Association, 2013). Investigators quantify the level of impulsivity with either self‐report questionnaires such as Barratt Impulsiveness Scale (BIS‐11; Patton, Stanford, & Barratt, 1995; Stanford et al., 2009) or laboratory paradigms, including delay discounting (Kirby, Petry, & Bickel, 1999; Matta, Gonçalves, & Bizarro, 2012; Reynolds & Schiffbauer, 2004) and continuous performance (Conners et al., 2000; Weafer, Baggott, & de Wit, 2013) tasks. The questionnaires and laboratory tasks characterize the behavioral scenarios manifesting impulsivity and identify the psychological constructs specific to task demand.
Impulsivity represents a multidimensional construct (Barratt & Slaughter, 1998; Nigg, 2000). The UPPS comprises subscales of urgency (U, tendency to act rashly when experiencing strong emotions), lack of perseverance (P, inability to act persistently), lack of premeditation (P, failure to plan or think before acting), and sensation seeking (S, seeking new and exciting experiences and sensations), aiming to capture multidimensional impulsivity traits (Whiteside, Lynam, Miller, & Reynolds, 2005). A revised version of the questionnaire, UPPS‐P, distinguishes the influences of negative versus positive affect (P) on urgency, leading to a 59‐item scale to address the five dimensions of impulsivity (Cyders & Smith, 2008; Cyders et al., 2007).
1.2. GMV correlates of impulsivity
Investigators have examined regional gray matter volumes (GMVs) as neural markers of impulsivity. For instance, higher GMV of the ventromedial prefrontal cortex was associated with higher impulsivity ratings in neurotypical children and adolescents (Boes et al., 2009). With the BIS‐11, some studies reported lower prefrontal cortical (PFC) but higher medial parieto‐occipital GMVs in association with impulsivity in healthy adults (Ide, Tung, Yang, Tseng, & Li, 2017; Korponay et al., 2017), and lower GMVs of dorsomedial PFC (dmPFC), insular cortex, orbitofrontal cortex, amygdala, and fusiform gyrus in association with impulsivity in developmentally typical adolescents (Du et al., 2016). Lower GMVs in the frontomedial cortex and insula and higher GMVs in the ventral striatum, hypothalamus, and anterior thalamus have been associated with greater temporal discounting (i.e., higher level of impulsivity) in neurotypical children (Mackey et al., 2017). Other studies reported higher striatal GMV in link with steeper discounting in neurotypical adults (Tschernegg et al., 2015) and smaller dmPFC GMV in correlation with higher negative urgency in neurotypical adults at 18–85 years of age (Muhlert & Lawrence, 2015). A more recent work on a cohort of 7–21 years old reported diminished PFC thickness in correlation with the UPPS‐P total score but no significant volumetric correlates in subcortical structures, such as the amygdala, hippocampus, and dorsal or ventral striatum (Merz, He, & Noble, 2018). Thus, while questionnaire assessments and laboratory tasks may help inform the underlying mechanism of impulsivity, the imaging findings of neural correlates appeared to vary across instruments and psychological constructs, suggesting the multidimensional nature of impulsivity. The findings also varied across age groups, raising the possibility that both genetic and environmental factors play important roles in shaping impulsivity.
1.3. Sex differences and heritability of impulsivity
Sex differences in impulsivity have been demonstrated in both adults and children, which may be related to higher prevalence of externalizing psychopathologies in males than in females (Hicks et al., 2007; Weinstein & Dannon, 2015; Zucker, 2008). Of the UPPS‐P traits, men scored higher than women on sensation seeking (Argyriou, Um, Wu, & Cyders, 2020; Billieux et al., 2012; Cyders, 2013; Van der Linden et al., 2006), lack of premeditation (Billieux et al., 2012; Pompeia et al., 2018), and lack of perseverance (Cyders, 2013; Van der Linden et al., 2006). In contrast, the findings on urgencies appear to be mixed, with some reporting higher negative and positive urgency in men (Pompeia et al., 2018) and others the opposite (Billieux et al., 2012).
Trait impulsivity is heritable (Kreek, Nielsen, Butelman, & LaForge, 2005), with the heritability (h 2) varying across studies with different instruments (Bezdjian, Baker, & Tuvblad, 2011). A longitudinal study showed moderate h 2 for inattention, motor, and nonplanning impulsivity of the BIS‐11, ranging from 0.33 to 0.56 for 11–13 years and from 0.19 to 0.44 for 14–16 years old (Niv, Tuvblad, Raine, Wang, & Baker, 2012). Delay discounting is commonly quantified with area under the discounting curve (AUC) and discounting rate (k) estimated from a hyperbolic model of performance (Odum, 2011). A study reported h 2 of 0.46 and 0.62 (AUC and k, respectively) at age 16 and of 0.35 and 0.55 at age 18 (Anokhin, Grant, Mulligan, & Heath, 2015). Thus, environmental influences may play an increasingly important role in determining impulsivity during late versus early adolescence. In adults, impulsivity quantified by the UPPS‐P total score was moderately heritable, with an h 2 = 0.49 (Gustavson et al., 2019). The heritability varies across the UPPS‐P subscores, with h 2 ~ 0.20 for positive and negative urgency, 0.64 for lack of premeditation, 0.40 for lack of perseverance, and 0.53 for sensation seeking, respectively (Tiego et al., 2020). However, no studies so far have examined the heritability of the neural correlates of dimensional impulsivity or sex differences in the heritability of the impulsivity traits or of their neural correlates.
1.4. The present work
The current study aimed at examining the volumetric correlates of dimensional impulsivity traits of the UPPS‐P as well as the sex differences and heritability of the behavioral traits and volumetric correlates in a large cohort of children in the Adolescent Brain Cognition Development (ABCD) project. A recent ABCD study focused on subcortical volumetric correlates of UPPS‐P subscores and, with many regional surface area and cortical thickness measures as covariates, highlighted lower right pallidum GMV and higher left caudate GMV in association with sensation seeking and positive urgency, respectively (Owens et al., 2020). Here, we employed whole‐brain voxel‐based morphometry to identify GMV markers of the five UPPS‐P subdomains and estimated the heritability of the volumetric markers in girls and boys combined as well as separately. We broadly hypothesized (1) significant sex differences in all domains of impulsivity, on the basis of extant literature; (2) distinct volumetric correlates of UPPS‐P subscores of impulsivity, and sex differences in the volumetric correlates; (3) higher heritability of the volumetric correlates than of the trait impulsivity measures, as genetics may dictate to a greater degree to neural versus behavioral phenotypes; and (4) sex differences in the volumetric correlates and heritability of correlates.
2. METHODS
2.1. Dataset: Subjects and assessment
The ABCD study investigates brain development and health of children longitudinally (https://abcdstudy.org). The current study employed the dataset of 11,601 children from the Release 2.0 cohort. Ninety‐nine children showed questionable image quality or poor image segmentation (see the last paragraph in Section 2.2 for details) and 28 did not complete the UPPS‐P scale or had incomplete UPPS‐P subscores, resulting in a final sample size of 11,474 (5,452 girls; age 9–10 years). We have obtained permission from the ABCD project to use the Open and Restricted Access data. All recruitment procedures and informed consents, including consent to share de‐identified data, were approved by the Institutional Review Board of the University of California San Diego.
The ABCD project employs a reduced 20‐item questionnaire abbreviated from the 40‐item youth version of UPPS‐P (Zapolski, Stairs, Settles, Combs, & Smith, 2010), following Cyders, Littlefield, Coffey, and Karyadi (2014). This shorter version maintains the original response format with most of the items conserved from the adults' form, allowing harmonization of data with other children's studies and transitioning to the adult's form as the ABCD children become adolescents (Barch et al., 2017). We extracted the UPPS‐P subscores from the Sum Scores Mental Health Youth instrument (abcd_mhy02), which indicated the total subscore by dimension as well as missing answers. The original response to each item can be found in the UPPS‐P for Children Short Form (abcd_upps01): negative urgency (upps7_y + upps11_y + upps17_y + upps20_y), lack of premeditation (upps6_y + upps16_y + upps23_y + upps28_y), lack of perseverance (upps15_y + upps19_y + upps22_y + upps24_y), sensation seeking (upps12_y + upps18_y + upps21_y + upps27_y), and positive urgency (upps35_y + upps36_y + upps37_y + upps39_y), totaling 20 items with 4 items per dimension. Each item's response is rated on a 1–4 scale, going from “strongly agree” to “strongly disagree” or vice versa, depending on the question. A higher score indicates higher impulsivity.
2.2. MRI and voxel‐based morphometry
Structural magnetic resonance imaging (MRI) data were acquired using optimized protocols for 3‐T machines, including Siemens Prisma, GE 750, and Philips, with voxel size 1‐mm isotropic, respectively (Casey et al., 2018). A customized 3‐T Siemens Connectome Skyra with a standard 32‐channel Siemens receiver head coil and a body transmission coil was used in the MRI scanning. T1‐weighted high‐resolution structural images were acquired using a three‐dimensional MPRAGE sequence with 1‐mm isotropic resolution (Field of view = 224 × 224 mm2, matrix = 320 × 320, 256 sagittal slices, Repetition time = 2,400 ms, Echo time = 2.14 ms, Inversion time = 1,000 ms, and Flip angle = 8°).
To perform voxel‐based morphometry (VBM) with appropriate templates for children, we constructed customized tissue probability maps (TPMs) and DARTEL templates (Ashburner, 2007) as well as an average T1 anatomical template for visualization. Briefly, a cohort of 1,000 ABCD children (500 girls) was selected from 10,000 random samples with 1,000 children (half girls) so that the cohort showed age and scan site distributions closest to those of the entire cohort. We used the function of Segment in the Statistical Parametric Mapping (SPM) software to generate individual's tissue maps and a TOM8 Toolbox (http://dbm.neuro.uni-jena.de/software/tom) to create population TPMs and a T1 anatomical template, controlling for the effects of age and sex (Wilke, Holland, Altaye, & Gaser, 2008). DARTEL templates were constructed using utilities available in SPM. This involved creating gray (rp1) and white (rp2) matter segments after affine registration followed by DARTEL nonlinear image registration, whereby all selected images were iteratively aligned with a template generated from their own mean and finally normalized to the Montreal Neurological Institute (MNI) space (International Consortium for Brain Mapping; ICBM template).
We implemented VBM to quantify regional GMVs with the CAT12 toolbox (http://dbm.neuro.uni-jena.de/vbm/). The details of the VBM analysis have been described earlier (Chen, Chaudhary, Wang, & Li, 2022; Ide et al., 2020). The VBM analysis identifies differences in the local composition of brain tissue, accounting for large‐scale variation in gross anatomy and location. The analysis includes spatially normalizing individuals' structural images to the same stereotactic space, segmenting the normalized images into distinct brain tissues, and smoothing the gray matter (GM) images. We used raw images to avoid potential interference with the CAT12 preprocessing pipeline. T1 images were first co‐registered to the MNI template using a multiple‐stage affine transformation during which 12 parameters were estimated. Co‐registration was performed with a coarse affine registration using mean square differences, followed by a fine affine registration using mutual information. Coefficients of the basis functions that minimized the residual squared difference between individual images and the template were estimated. Our custom TPMs constructed from 1,000 ABCD children were used in the initial affine transformation. T1 images were then preprocessed with spatial‐adaptive nonlocal means denoising filters (Manjon, Coupe, Marti‐Bonmati, Collins, & Robles, 2010) as well as Markov random fields, corrected for intensity bias field, and segmented into cerebrospinal fluid, GM, and white matter (Rajapakse, Giedd, & Rapoport, 1997). Segmented and initially registered tissue class maps were normalized using DARTEL (Ashburner, 2007), a fast diffeomorphic image registration algorithm of SPM. As a high‐dimensional nonlinear spatial normalization method, DARTEL generates mathematically consistent inverse spatial transformations. We used our custom DARTEL template in MNI space, as constructed from 1,000 ABCD children, to drive DARTEL normalization. Skull‐stripping and final cleanup (to remove remaining meninges and correct for volume effects in some regions) were performed with default parameters in the CAT12. In particular, skull‐stripping was performed by refining the probability tissue maps of SPM using adaptive probability region‐growing. Normalized GM maps were modulated to obtain the absolute volume of GM tissue corrected for individual brain sizes. Finally, the GM maps were smoothed by convolving with an isotropic Gaussian kernel (Full width at half maximum = 8 mm). In addition, total intracranial volume (TIV) of each participant was estimated and used in the second‐level analyses as a covariate to correct for brain sizes.
Quality check of images was performed visually and quantitatively with tools available in the CAT12 (Gaser & Dahnke, 2016). One axial slice (z = 0) per subject was plotted and visually checked (option “Display slices”), and outliers were detected by computing the voxel‐wise cross‐correlation of GM density across subjects (option “Check sample homogeneity”). A total of 47 subjects presented clearly faulty segmentation of brain tissues and were removed from group analyses. The faulty segmentation likely resulted from poor contrast or artifacts of the structural images or abnormal brain shapes. Additionally, for each subject, pairwise correlations were computed between the subject's GMV and all the other subjects' GMV. The mean correlation represented how similar the subject's GMV was to the rest of the sample. A total of 52 subjects with a mean correlation < 0.70, suggesting higher variance of the GM densities, were also removed.
2.3. Group data analyses
We performed whole‐brain linear regressions against all UPPS‐P subscores, with age in months, TIV, study site, and scanner manufacturer/model as covariates in the same model in girls and boys combined as well as separately. We evaluated the results with a voxel p < .05 corrected for family‐wise error (FWE) of multiple comparisons on the basis of Gaussian random field theory as implemented in the SPM. The extent threshold for a cluster to meet the cluster level p < .05 FWE‐corrected was 131 voxels. However, as investigators have also argued that with a voxel p < .05, FWE‐corrected, even clusters smaller than the extent threshold should be reported to reflect spatial specificity (Nichols, 2012; Woo, Krishnan, & Wager, 2014), we have opted to show more clusters with an extent threshold set at 32 voxels. Thus, clusters with the minimal size of 32 voxels were overlaid on the custom MRI template obtained from the 1,000 ABCD children. We hope that reporting the findings in detail would facilitate future meta‐analyses. Effect sizes were computed using tools available in the CAT12, by approximating Cohen's d (Cohen, 2013) from the t‐statistics using the expression (Kleber et al., 2016). It is beneficial to use Cohen's d for conceptualizing and comparing the size of a difference independently of the specific measure used, which enables comparisons between studies concerned with the same factor but using different dependent measures (Fritz, Morris, & Richler, 2012).
To examine how the GMV correlates were shared or nonshared across the componential impulsivity traits, we performed inclusive (e.g., lack of premeditation and sensation seeking) and exclusive [e.g., lack of premeditation and (~sensation seeking)] masking to identify the shared and nonshared correlates. We tested the hypothesis that the nonshared relative to the shared GMVs would be more strongly correlated with the trait scores. We performed these analyses on the volumetric correlates identified from all subjects and examined the differences in the correlations using slope tests (Zar, 1999) with age, sex, TIV, study site, and scanner manufacturer/model as covariates and showed two‐tailed p values.
For the regions of interest (ROIs) identified from linear regressions in girls and boys combined or alone, we examined sex differences in the correlations using slope tests with age, TIV, study site, and scanner manufacturer/model as covariates and showed two‐tailed p values. Note that the analyses did not represent “double‐dipping,” as the slope tests may confirm or refute sex differences (Chen, Li, Ide, Luo, & Li, 2021; Dhingra et al., 2020; Ide et al., 2020; Le et al., 2019; Li et al., 2020, 2021). This was because the regression maps were identified with a threshold and a cluster showing correlation in boys could show a correlation that just missed the threshold in girls and vice versa. Thus, slope tests were needed to examine whether the correlations were indeed different between sexes. Additionally, we conducted ROI analyses by including race/ethnicity, family income, and highest education of parents as additional covariates and showed the results in the Supporting Information.
2.4. Heritability of UPPS‐P scores and volumetric correlates
Of the 11,474 children, 634 and 2,816 were identified as monozygotic twins (MZ) and dizygotic twins/siblings (DZ), respectively. For girls and boys combined and separately, we used Mplus 8 (Muthen & Muthen, 2012) to compute the heritability (i.e., genetic influence), shared environmental influence, and unique environmental influence (Visscher, Hill, & Wray, 2008; Visscher et al., 2006) for the UPPS‐P subscores as well as the volumetric correlates on the basis of univariate ACE models. Only the same‐sex DZ pairs were included in the analyses of girls and boys separately. The ACE model decomposes the observed variance into additive genetic factors (A), also known as heritability, shared environmental factors (C), and unique environmental factors (E), in addition to measurement errors (Kohler, Behrman, & Schnittker, 2011). The correlation in A is fixed to 1.0 for MZ and 0.5 for DZ. The correlation in C is set to 1.0 for both MZ and DZ based on the equal environment assumption. The correlation in E is set to zero. The expected variance–covariance matrices within MZ and DZ are as follows:
where a, c, and e represent the path coefficients for the A, C, and E factors, respectively (Rijsdijk & Sham, 2002). The variance of A, C, and E was estimated using the maximum likelihood method on the variance–covariance matrices, and 95% confidence intervals (CIs) of A, C, and E were computed: 95% CI = mean ± 1.96 × standard error, with the assumption that the population standard deviation is a known value. Values of χ 2/df, root‐mean‐square error of approximation (RMSEA), and Tucker–Lewis index (TLI) were used as model fit indices. A χ 2/df < 2, an RMSEA < 0.06, or a TLI > 0.95 indicates good fit. Genetic variance (a 2) < 0.30, 0.30–0.60, and >0.60 is considered low, moderate, and high, respectively.
We used a 2 (sex) × 5 (UPPS‐P subdomain) mixed‐model analysis of variance (ANOVA) to compare the a 2 of UPPS‐P subscores. We used repeated‐measures ANOVA to compare the a 2 of the volumetric correlates of lack of premeditation, sensation seeking, and positive urgency identified of girls and boy combined. The a 2 of volumetric correlates that were identified of sensation seeking and positive urgency for both girls and boys separately was also examined with a 2 (sex) × 2 (subdomain) mixed‐model ANOVA. Following these ANOVAs, we performed pairwise comparisons and evaluated the results with a p value corrected for the number of comparisons.
3. RESULTS
3.1. UPPS‐P subscores
Figure 1 shows the distribution of the UPPS‐P subscores and pairwise correlations between the subscores across all subjects. Notably, although none of subscores showed significant skewness (all ≤ 0.86), sensation seeking (skewness = 0.03) best followed a normal distribution. All pairwise correlations were positive except for sensation seeking versus lack of perseverance (r = −.10, p < .001), as also reported previously in children (Marmorstein, 2013) and adults (Muller, Langner, Cieslik, Rottschy, & Eickhoff, 2015; Tiego et al., 2020). Positive urgency and negative urgency (r = .49, p < .001) as well as lack of perseverance and lack of premeditation (r = .45, p < .001) were strongly correlated. With age in months as a covariate, two sample t‐tests showed significantly higher UPPS‐P subscores in boys as compared to girls (Table 1), indicating greater impulsivity across all subdomains in boys.
FIGURE 1.

Distribution of UPPS‐P subscores and intercorrelations. NegUr, LackPM, LackPer, SS, and PosUr represent negative urgency, lack of premeditation, lack of perseverance, sensation seeking, and positive urgency, respectively. All UPPS‐P subscores were positively correlated (r's = .06–.49), except that lack of perseverance was negatively correlated with sensation seeking (r = −.10). Grey dashed lines represent 95% confidence intervals of the mean regressions (black solid lines)
TABLE 1.
Sex differences in the UPPS‐P subscores
| Subscore | Girls | Boys | t | p |
|---|---|---|---|---|
| Negative urgency | 8.25 ± 2.64 | 8.70 ± 2.63 | −9.16 | <.001 |
| Lack of premeditation | 7.46 ± 2.32 | 8.01 ± 2.40 | −12.55 | <.001 |
| Lack of perseverance | 6.90 ± 2.26 | 7.17 ± 2.24 | −6.27 | <.001 |
| Sensation seeking | 9.43 ± 2.65 | 10.12 ± 2.67 | −13.75 | <.001 |
| Positive urgency | 7.76 ± 2.94 | 8.19 ± 2.94 | −7.85 | <.001 |
Note: Values of mean ± SD; t and p values are based on two sample t‐tests with age in months as a covariate.
3.2. GMV correlates of UPPS‐P subscores
We examined regional volumetric correlates of each UPPS‐P subscore in a linear whole‐brain regression with all five subscores as regressors and age in months, TIV, study site, and scanner manufacturer/model as covariates. We performed the regressions for girls and boys combined (with sex as an additional covariate) and separately and evaluated the results at a voxel p < .05 FWE‐corrected. Brain regions with GMVs in correlation with lack of premeditation, sensation seeking, and positive urgency are shown in Figures 2, 3, 4, respectively, and the clusters are summarized in Tables S1–S3. At the same threshold, no GMV clusters were identified of negative urgency or of lack of perseverance in girls and boys combined or separately.
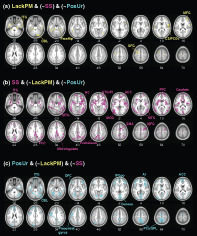
FIGURE 2.
Gray matter volume correlates of lack of premeditation for (a) all, (b) girls, and (c) boys. Nil represents no significant findings. Color bars show voxel T and Cohen's d values. Warm colors indicate positive correlations. No clusters showed significant negative correlations. Neurological orientation: right = right. These clusters are summarized in Table S1
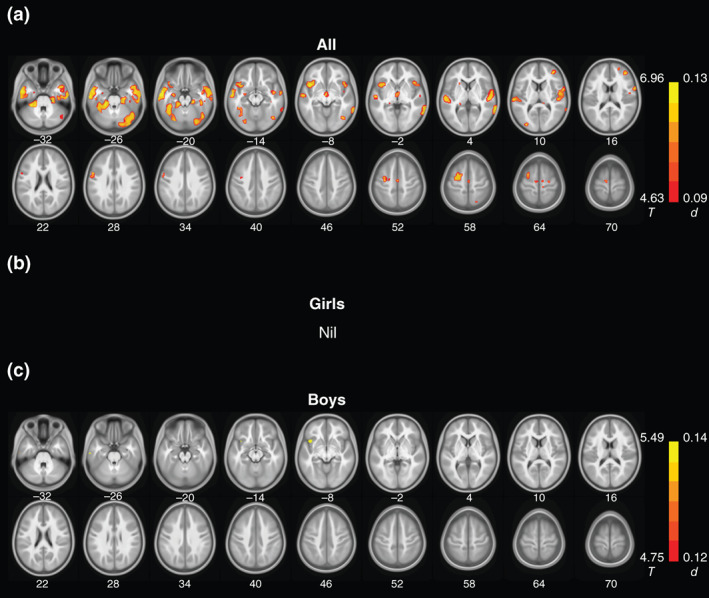
FIGURE 3.
Gray matter volume correlates of sensation seeking for (a) all, (b) girls, and (c) boys. Color bars show voxel T and Cohen's d values. Warm colors indicate positive correlations. No clusters showed significant negative correlations. Neurological orientation: right = right. These clusters are summarized in Table S2
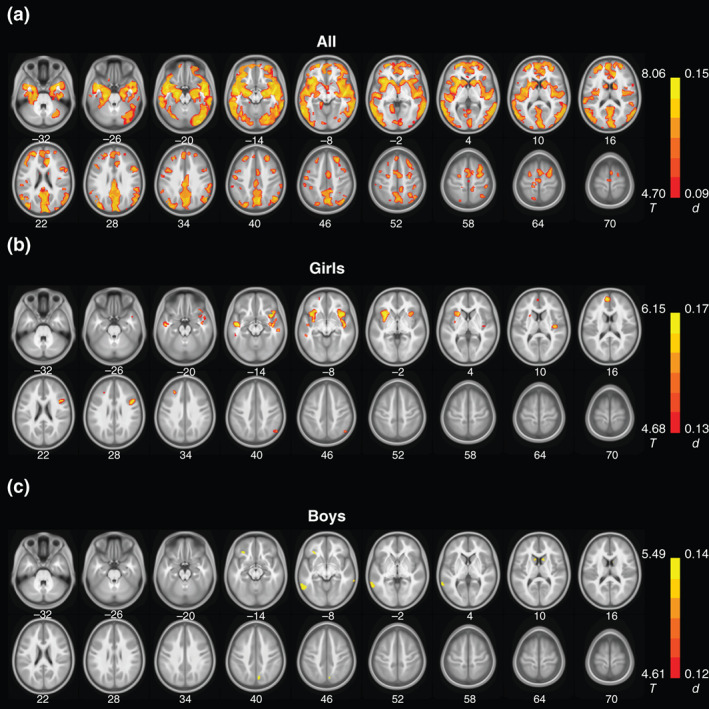
FIGURE 4.

Gray matter volume correlates of positive urgency for (a) all, (b) girls, and (c) boys. Color bars show voxel T and Cohen's d values. Cold colors indicate negative correlations. No clusters showed positive correlations. Neurological orientation: right = right. These clusters are summarized in Table S3
We have also performed whole‐brain linear regressions in five separate models, each with one of the UPPS‐P subscores as a regressor and the same covariates. The results are shown in Figure S1A–E.
3.2.1. GMV correlates of lack of premeditation
Higher levels of lack of premeditation were associated with larger GMVs of a wide array of cortical and subcortical regions bilaterally, including insula, frontal, temporal, and visual cortices, thalamus, hippocampus, and cerebellum in girls and boys combined. Girls alone did not show any significant clusters; boys alone showed higher GMV of a small cluster in the left insula in correlation with lack of premeditation. These clusters are shown in Figure 2 and summarized in Table S1.
3.2.2. GMV correlates of sensation seeking
Across all subjects, sensation seeking was positively correlated with GMVs of many brain regions, too, including bilateral insula, frontal, parietal, temporal, and occipital cortices, posterior cingulate, precuneus, and a number of subcortical structures, including bilateral caudate, putamen, and thalamus. In girls alone, higher levels of sensation seeking were associated with larger GMVs of bilateral insula and superior temporal gyri, left putamen, and right middle frontal gyrus. In boys alone, greater sensation seeking was associated with higher GMVs of small clusters of right middle temporal gyrus and bilateral caudate head. These clusters are shown in Figure 3 and summarized in Table S2.
3.2.3. GMV correlates of positive urgency
In girls and boys combined, positive urgency was negatively correlated with GMVs of bilateral insula, lateral and medial orbitofrontal cortex, anterior cingulate cortex, supplementary motor area, superior/middle/inferior temporal cortex, hippocampus/parahippocampal gyrus, amygdala, posterior cingulate/precuneus, occipital cortex, and dorsal striatum. Examined separately, boys and girls overall shared a similar pattern in left precentral gyrus, bilateral middle/inferior temporal and fusiform gyri, and left amygdala with smaller GMVs in association with higher levels of positive urgency. In addition, girls appeared to show smaller GMVs of bilateral middle/superior temporal and lateral occipital cortex, posterior cingulate cortex, and precuneus; and boys appeared to show smaller GMVs of bilateral orbitofrontal cortex, insula, putamen, caudate, and supplementary motor area, in correlation with positive urgency. These clusters are shown in Figure 4 and summarized in Table S3.
3.3. Shared and nonshared GMV correlates of UPPS‐P subscores
Across lack of premeditation, sensation seeking, and positive urgency, some of the volumetric correlates overlapped and some did not (Figure S2).
For the GMVs that overlapped, the correlations could go in the same or opposite direction. We extracted the GMV estimates of these “overlapping clusters” and visualized the patterns of correlations in Figure 5, with age in months, sex, TIV, study site, and scanner manufacturer/model as covariates. Bilateral temporal cortex, left anterior insula, right cerebellum, and hypothalamus showed GMVs in positive correlations with both lack of premeditation (r = .04, p < .001) and sensation seeking (r = .05, p < .001). Bilateral temporal cortex, left anterior insula, and right cerebellum also showed GMVs each in positive correlations with lack of premeditation (r = .04, p < .001) and in negative correlations with positive urgency (r = −.09, p < .001). An extensive array of brain regions, including bilateral insula, medial and lateral orbitofrontal cortex, temporal and occipital‐temporal cortices, caudate head, precuneus, left amygdala, left angular gyrus, and right cerebellum showed GMVs each in positive correlations with sensation seeking (r = .05, p < .001) and in negative correlations with positive urgency (r = −.09, p < .001). These results are shown in Figure S3. We also performed the same correlation analyses with race/ethnicity, family income, and highest parents' education as additional covariates. The results are shown in Table S5.
FIGURE 5.

Shared gray matter volume (GMV) correlates across subscores and the correlations of GMVs of these clusters (in green) with UPPS‐P subscores. (a) LackPM & SS, (b) LackPM & PosUr, and (c) SS & PosUr. Note that the residual values after controlling for age, sex, total intracranial volume (TIV), study site, and scanner manufacturer/model are shown here. Dashed lines represent 95% confidence intervals of the mean regressions (solid lines). ACC, anterior cingulate cortex; AI, anterior insula; CBL, cerebellum; ITG, inferior temporal gyrus; LackPM, lack of premeditation (in yellow); MTG, middle temporal gyrus; PosUr: positive urgency (in cyan); SS, sensation seeking (in magenta); STG, superior temporal gyrus. ***p < .001
For the volumetric correlates that did not overlap, lack of premeditation involved specifically a few small clusters in bilateral temporal cortices, including the hippocampus and parahippocampus. Sensation seeking involved bilateral caudate head, mid‐ and posterior cingulate, inferior parietal, inferior/middle temporal, and superior/middle frontal cortices. Positive urgency involved the precuneus, precentral cortex, lateral orbitofrontal cortex, and medial temporal cortex (Figure 6). For these nonshared GMV correlates (Figure S4A–C), the correlations with impulsivity subscores were stronger as compared to the shared GMV correlates, although slope tests did not reveal significant differences in slopes between the regressions (Table S4). We also performed the same correlation analyses with race/ethnicity, family income, and highest parents' education as additional covariates. The results are shown in Table S5.
FIGURE 6.
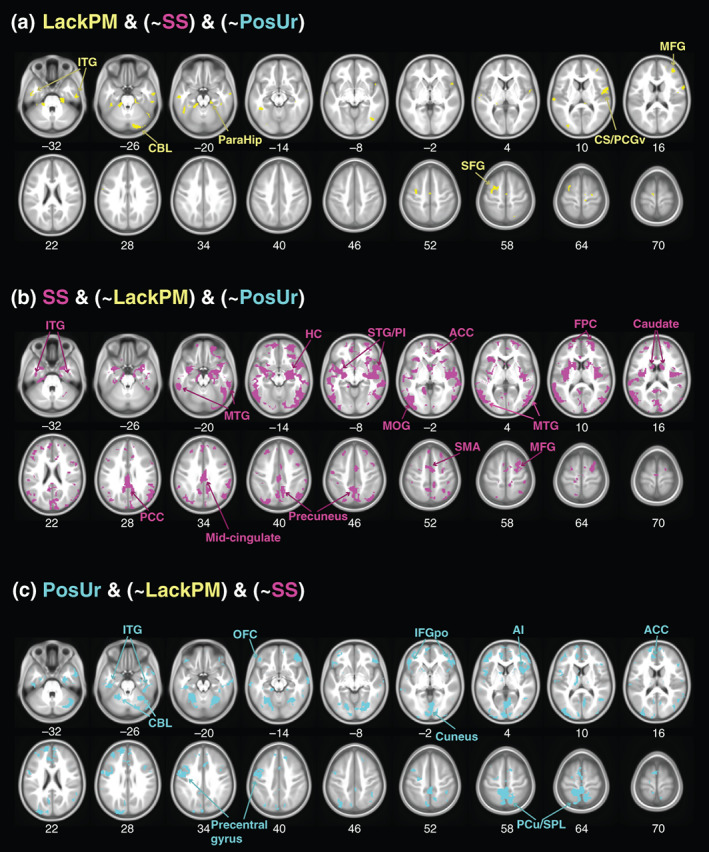
Nonshared gray matter volume (GMV) correlates of UPPS‐P subscores in girls and boys combined. (a) LackPM & (~SS) & (~PosUr); (b) SS & (~LackPM) & (~PosUr); and (c) PosUr & (~LackPM) & (~SS). ACC, anterior cingulate cortex; CBL, cerebellum; CS/PCGv, central sulcus/precentral gyrus, ventral; FPC, frontopolar cortex; HC, hippocampus; IFGpo, inferior frontal gyrus, pars orbitalis; ITG, inferior temporal gyrus; LackPM, lack of premeditation (in yellow); MFG, middle frontal gyrus; mid‐cingulate, middle cingulate cortex; MTG, middle temporal gyrus; OFC, orbitofrontal cortex; ParaHip, parahippocampal gyrus; PCC, posterior cingulate cortex; PCu/SPL, precuneus/superior parietal lobule; PI, posterior insula; PosUr, positive urgency (in cyan); SFG, superior frontal gyrus; SMA, supplementary motor area; SS, sensation seeking (in magenta); STG, superior temporal gyrus
We further examined whether these nonshared correlates of lack of premeditation showed a stronger correlation with lack of premeditation than with sensation seeking subscore and vice versa, with age in months, sex, TIV, study site, and scanner model as covariates. The results showed that the nonshared GMV correlates of lack of premeditation showed stronger correlation with lack of premeditation (r = .045, p < .001) than with sensation seeking (r = .024, p = .009) subscore, although the difference in the slope of regression was only marginally significant (t = 1.83, p = .068). The nonshared correlates of sensation seeking showed stronger correlation with sensation seeking (r = .050, p < .001) than with lack of premeditation (r = .016, p = .089) subscore, and the two regressions differed significantly in slope (t = 2.30, p = .021). We performed the same analyses in contrasting lack of premeditation and sensation seeking with positive urgency. Because the GMVs correlates went in opposite directions, the slope tests were all significant, as expected (Table 2). We also performed the same analyses with race/ethnicity, family income, and highest parents' education as additional covariates. The results are shown in Table S6.
TABLE 2.
Correlations between GMVs of nonshared clusters and subscores and the statistics of slope tests
| LackPM | SS | PosUr | Slope test | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | t | p | |
| LackPM & (~SS) | .045 | <.001 | .024 | .009 | / | / | 1.83 | .068 |
| LackPM & (~PosUr) | .048 | <.001 | / | / | −.068 | <.001 | 8.59 | <.001 |
| SS & (~LackPM) | .016 | .089 | .050 | <.001 | / | / | 2.30 | .021 |
| SS & (~PosUr) | / | / | .052 | <.001 | −.068 | <.001 | 8.99 | <.001 |
| PosUr & (~LackPM) | .018 | .057 | / | / | −.097 | <.001 | 8.00 | <.001 |
| PosUr & (~SS) | / | / | .026 | .006 | −.102 | <.001 | 9.44 | <.001 |
Note: Correlations were computed with age in months, sex, TIV, study site, and scanner model as covariates.
Abbreviations: GMV, gray matter volume; LackPM, lack of premeditation; PosUr, positive urgency; SS, sensation seeking; TIV, total intracranial volume.
3.4. Sex‐specific GMV correlates of UPPS‐P subscores
We combined the clusters identified from whole‐brain regressions of each subscore as “joint ROIs” and extracted the GMV estimates of the joint ROIs. We performed slope tests to examine sex differences in the correlations between the GMV estimates and UPPS‐P subscores. Girls as compared to boys showed more significantly positive correlations between the GMVs of ROIs identified of sensation seeking in girls (t = 3.81, p < .001). Girls relative to boys also showed more significantly negative correlations between the GMVs of ROIs identified of positive urgency in girls (t = 2.99, p = .003). No other correlations showed significant slope differences, considering multiple comparisons (p = .05/8). Table 3 summarizes the statistics of the regressions in girls and boys and of the slope tests of sex difference in the regressions. We also performed the same analyses with race/ethnicity, family income, and highest parents' education as additional covariates. The results are presented in Table S7.
TABLE 3.
Sex differences in the correlations between UPPS‐P subscores and GMVs
| Joint ROIs identified from whole‐brain regressions for | Girls | Boys | Slope test | |||
|---|---|---|---|---|---|---|
| r | p | r | p | t | p | |
| Lack of premeditation | ||||||
| All | .04 | .007 | .04 | .002 | 0.00 | .998 |
| Boys | .02 | .094 | .05 | <.001 | 1.59 | .113 |
| Sensation seeking | ||||||
| All | .07 | <.001 | .04 | <.001 | 1.80 | .072 |
| Girls | .10 | <.001 | .03 | .011 | 3.81 | <.001* |
| Boys | .05 | <.001 | .06 | <.001 | 0.53 | .594 |
| Positive urgency | ||||||
| All | −.13 | <.001 | −.10 | <.001 | 2.06 | .040 |
| Girls | −.14 | <.001 | −.10 | <.001 | 2.99 | .003* |
| Boys | −.11 | <.001 | −.11 | <.001 | 0.87 | .386 |
Note: Joint ROIs: all clusters identified from the regression were combined into a single mask. Correlations were computed with age in months, TIV, study site, and scanner model as covariates. *p values significant with correction for a total of 8 comparisons (.05/8 = .0063).
Abbreviations: GMV, gray matter volume; ROIs, regions of interest; TIV, total intracranial volume.
3.5. Heritability of UPPS‐P subscores and volumetric correlates
Table 4 shows the heritability and shared and nonshared environmental effects of UPPS‐P subscores as well as the volumetric correlates, where identified, in boys and girls combined and separately. The ACE models showed good model fit for the great majority of the measures (Table S8). For boys and girls combined and alone, lack of perseverance was moderately heritable (a 2 = 0.43–0.47), whereas other subscores were mostly weakly heritable (a 2 = 0.06–0.32). The shared environmental effects on most of the UPPS‐P subscores were negligible (c 2 = 0.00–0.18). All volumetric correlates identified from whole‐brain regressions in boys and girls combined and alone were moderately to highly heritable (a 2 = 0.42–0.84).
TABLE 4.
Genetic, shared environmental and nonshared environmental effects on UPPS‐P subscores and volumetric correlates
| Variable | UPPS‐P subscores | Volumetric correlates | ||||
|---|---|---|---|---|---|---|
| a 2 (A) | c 2 (C) | e 2 (E) | a 2 (A) | c 2 (C) | e 2 (E) | |
| All (MZ: 317 pairs, DZ: 1,408 pairs) | ||||||
| NegUr | 0.22 [−0.01, 0.44] | 0.07 [−0.07, 0.21] | 0.71 [0.61, 0.82] | NA | NA | NA |
| LackPM | 0.21 [0.13, 0.28] | 0.00 [0.00, 0.00] | 0.79 [0.72, 0.87] | 0.76 [0.69, 0.83] | 0.18 [0.11, 0.25] | 0.06 [0.05, 0.07] |
| LackPer | 0.44 [0.37, 0.52] | 0.00 [0.00, 0.00] | 0.56 [0.49, 0.63] | NA | NA | NA |
| SS | 0.28 [0.06, 0.51] | 0.02 [−0.12, 0.16] | 0.70 [0.59, 0.80] | 0.84 [0.76, 0.91] | 0.11 [0.04, 0.19] | 0.05 [0.04, 0.06] |
| PosUr | 0.24 [0.17, 0.32] | 0.00 [−0.003, 0.003] | 0.76 [0.69, 0.83] | 0.80 [0.73, 0.87] | 0.15 [0.08, 0.22] | 0.05 [0.04, 0.05] |
| Girls (MZ: 160 pairs, DZ: 466 pairs) | ||||||
| NegUr | 0.24 [−0.08, 0.56] | 0.07 [−0.14, 0.29] | 0.69 [0.55, 0.82] | NA | NA | NA |
| LackPM | 0.12 [−0.24, 0.48] | 0.06 [−0.17, 0.30] | 0.82 [0.66, 0.97] | NA | NA | NA |
| LackPer | 0.43 [0.32, 0.55] | 0.00 [0.00, 0.00] | 0.57 [0.45, 0.69] | NA | NA | NA |
| SS | 0.11 [−0.22, 0.45] | 0.16 [−0.07, 0.38] | 0.73 [0.58, 0.88] | 0.63 [0.49, 0.77] | 0.21 [0.08, 0.35] | 0.16 [0.12, 0.19] |
| PosUr | 0.17 [0.06, 0.28] | 0.00 [−0.001, 0.001] | 0.83 [0.72, 0.94] | 0.65 [0.54, 0.76] | 0.28 [0.16, 0.39] | 0.07 [0.06, 0.09] |
| Boys (MZ: 157 pairs, DZ: 501 pairs) | ||||||
| NegUr | 0.06 [−0.28, 0.40] | 0.18 [−0.04, 0.40] | 0.76 [0.61, 0.92] | NA | NA | NA |
| LackPM | 0.22 [0.11, 0.34] | 0.00 [0.00, 0.00] | 0.78 [0.66, 0.89] | 0.42 [0.26, 0.57] | 0.33 [0.20, 0.46] | 0.25 [0.20, 0.32] |
| LackPer | 0.47 [0.37, 0.58] | 0.00 [0.00, 0.00] | 0.53 [0.42, 0.63] | NA | NA | NA |
| SS | 0.29 [0.17, 0.40] | 0.00 [−0.001, 0.001] | 0.71 [0.60, 0.83] | 0.47 [0.28, 0.65] | 0.22 [0.07, 0.37] | 0.31 [0.24, 0.39] |
| PosUr | 0.32 [0.21, 0.43] | 0.00 [−0.001, 0.001] | 0.68 [0.57, 0.79] | 0.60 [0.50, 0.70] | 0.34 [0.24, 0.44] | 0.06 [0.05, 0.08] |
Note: Only same‐sex DZ twins/siblings were included in the girls and boys separately. a 2 represents proportion of variance due to additive genetic effects (A); c 2 represents proportion of variance due to shared environmental effects (C); e 2 represents proportion of variance due to nonshared environmental effects (E); and the values in square brackets are 95% confidence intervals.
Abbreviations: DZ, dizygotic/sibling; LackPer, lack of perseverance; LackPM, lack of premeditation; MZ, monozygotic; NA, not available; NegUr, negative urgency; PosUr, positive urgency; SS, sensation seeking.
A 2 (sex) × 5 (subdomain) mixed‐effects ANOVA of the heritability of UPPS‐P subscores showed significant sex and subdomain main effects [F(1, 1282) = 1,397.21 and F(4, 5128) = 10,720.51, respectively; both p's < .001] as well as a significant interaction effect [F(4, 5128) = 1,692.31, p < .001]. Simple effect tests showed significant sex differences (girls vs. boys) in the heritability of negative urgency (a 2 = 0.24 vs. 0.06) and lack of premeditation (0.12 vs. 0.22), with both p's < .001. Across both sexes, the heritability differed pairwise across all UPPS‐P subdomains (p's ≤ .042, uncorrected) except for negative urgency versus lack of premeditation (0.15 vs. 0.17, p = .096) and lack of premeditation versus positive urgency (0.17 vs. 0.17, p = 1.000). In girls alone, the heritability differed pairwise across UPPS‐P subdomains (p's ≤ .001) except for lack of premeditation versus sensation seeking (0.12 vs. 0.11, p = .632). In boys alone, the heritability differed pairwise across all UPPS‐P subdomains (p's ≤ .008).
The repeated‐measures ANOVA on the heritability of the volumetric correlates of lack of perseverance, sensation seeking, and positive urgency for girls and boys combined showed a significant main effect [F(2, 3448) = 1,206.14, p < .001]. Pairwise comparisons confirmed significant differences in the heritability for all these volumetric correlates (p's ≤ .001).
A 2 (sex) × 2 (subdomain: sensation seeking, positive urgency) mixed‐effects ANOVA of the heritability of volumetric correlates showed that the main effects of sex and subdomain [F(1, 1282) = 5,229.40 and F(1, 1282) = 319,035.50, respectively; p's < .001] as well as the interaction effect [F(1, 1282) = 2,810.26, p < .001] were all significant. Simple effect tests showed significant sex differences (girls vs. boys) in the heritability of volumetric correlates of sensation seeking (0.63 vs. 0.47) and positive urgency (0.65 vs. 0.60), with p's < .001. The differences in the heritability of volumetric correlates of the two subdomains were significant in boys only (SS = 0.47 vs. PosUr = 0.60, p < .001). Across both sexes, the heritability for the volumetric correlates of positive urgency was significantly higher than that of sensation seeking (0.63 vs. 0.55, p < .001).
4. DISCUSSION
The UPPS‐P dimensional impulsivity traits involve both shared and nonshared volumetric correlates. Higher levels of lack of premeditation and sensation seeking were correlated with larger GMVs of many cortical and subcortical regions, including the hypothalamus. In contrast, higher positive urgency was correlated with smaller GMVs of many of the same regions. In nonshared correlates, sensation seeking implicates bilateral caudate head and mid‐cingulate cortex and positive urgency implicates bilateral lateral orbitofrontal cortex and left precentral gyrus. Boys relative to girls scored higher in all impulsivity dimensions. However, girls relative to boys showed significantly stronger positive and negative correlations between sensation seeking and insula, putamen, and inferior frontal gyrus (IFG) GMVs and between positive urgency and cingulate cortex, insula, and IFG GMVs, respectively. The dimensional impulsivity traits were weakly to moderately heritable with lack of perseverance showing the highest a 2 in both girls and boys. The GMV correlates were highly heritable in girls and boys combined, with those of sensation seeking and positive urgency showing the highest a 2 in girls and in boys, respectively. We highlight the main findings in discussion.
4.1. Relationships between UPPS‐P subscores
All UPPS‐P subscores were positively correlated, with an r ranging from .06 to .49, except for lack of perseverance and sensation seeking, which were negatively correlated (r = −.10). Previous studies have likewise largely reported positive correlations between UPPS‐P subscores but also noted lack of correlation between sensation seeking and urgency in adults (Tiego et al., 2020) or between sensation seeking and lack of premeditation in children (Marmorstein, 2013) or adults (Muller et al., 2015), as well as negative correlations of lack of premeditation with positive urgency and with sensation seeking in young adults (Carlson, Pritchard, & Dominelli, 2013). Thus, sensation seeking appeared to be a more distinct impulsivity trait. Indeed, by including other assessments of disinhibition and reward and punishment sensitivity, the latter study showed that while positive urgency and sensation seeking are both positively correlated with reward sensitivity and disinhibition, sensation seeking is unique in showing a negative correlation with punishment sensitivity (Carlson et al., 2013). In an earlier study of 481 young adults with the UPPS questionnaire, all four subscores were positively correlated with externalizing problems, including crime and delinquency, aggression, as well as alcohol and drug use (Miller, Flory, Lynam, & Leukefeld, 2003). In contrast, all but sensation seeking was positively correlated with internalizing problems, including anxiety and depression. The current along with these earlier findings suggest that relative to other impulsivity traits, sensation seeking is associated with the tendency to engage in new and thrilling experiences without incurring anxiety, depression, or consideration of punishing consequences. These features may aid in distinguishing sensation seeking from other impulsivity traits.
4.2. Volumetric correlates of dimensional impulsivity traits
Regional GMVs were related to impulsivity traits in both positive and negative directions. Higher levels of lack of premeditation and sensation seeking were both associated with greater GMVs of the left insula, bilateral temporal cortices and cerebellum, left superior frontal and right angular gyri, and hypothalamus. A recent ABCD study focused on subcortical volumetrics and highlighted smaller right pallidum GMV in association with sensation seeking (Owens et al., 2020); however, the study examined the volumetric correlates by including regional measures of cortical thickness and surface area as covariates, which may have influenced the findings. Earlier studies of developmentally typical adolescents showed higher trait impulsivity in correlation with greater GMVs of the precentral gyrus and temporal cortex (Schilling et al., 2013) and dmPFC, insula, OFC, amygdala, and fusiform gyrus (Du et al., 2016). More aggressive and impulsive behaviors were associated with larger striatal volumes in neurotypical children (Ducharme et al., 2011). We recently reported that behavioral activation system traits were positively correlated with GMVs of the ventral striatum, cerebellar vermis, and parahippocampal gyrus (Ide et al., 2020). Thus, the current findings add to this literature by demonstrating higher GMVs specifically in association with lack of premeditation and sensation seeking but not with other impulsivity traits. The brain undergoes extensive synaptic pruning to maintain normal functions during adolescence (Grigg‐Damberger & Ralls, 2013; Paus, Keshavan, & Giedd, 2008). It is possible that delayed synaptic pruning during development may account for larger regional GMVs in link with behavioral impulsivity. On the other hand, this explanation unlikely accounts for GMV correlates of all components of impulsivity, as we observed smaller regional GMVs in association with higher positive urgency.
We observed that higher positive urgency—emotion‐based rash behaviors—was associated with lower GMVs of many cortical and subcortical regions. In contrast, Owens et al. (2020) reported with ABCD data higher caudate GMV in association with positive urgency; again, it is not clear whether this discrepancy may have to do with the inclusion of other morphometric measures as covariates in the analyses of Owens et al. Many of these regions, such as the dorsolateral PFC and temporal pole, support emotional regulation (Muhlert & Lawrence, 2015). Urgency represents a major determinant of maladaptive behaviors such as drinking and gambling (Berg, Latzman, Bliwise, & Lilienfeld, 2015; Billieux, Gay, Rochat, & Van der Linden, 2010; Johnson, Carver, & Joormann, 2013; Selby, Anestis, & Joiner, 2008). Elevated positive mood may compromise inhibitory control (Dekker & Johnson, 2018; Johnson, Tharp, Peckham, Sanchez, & Carver, 2016) and evaluation of behavioral consequences, as in drug use and risky sex (Cyders & Smith, 2008; Zapolski, Cyders, & Smith, 2009). An earlier study showed that positive but not negative urgency was associated with lower frontostriatal circuit activity during rewarded antisaccades, indicating compromised impulse control under an incentivizing condition (Tervo‐Clemmens et al., 2017). Notably, although we did not observe GMV correlates of negative urgency here, higher negative urgency was associated with lower GMVs of bilateral globus pallidum, right precentral gyrus, and left IFG when UPPS‐P subscores were modeled separately (see Supporting Information). Positive and negative urgency were highly correlated (r = 0.49), and prior research has shown similar neuroanatomical correlates of negative and positive urgency (Johnson, Elliott, & Carver, 2020; Owens et al., 2020). The current findings suggest positive relative to negative urgency as a more dominant trait in underscoring the volumetric markers of impulsivity.
4.3. Shared and nonshared volumetric correlates of dimensional impulsivity traits
Including all five subscores in the model provides a better fit to the data; however, this was not mean to “control for” the mutual influences of component impulsivity traits (Miller & Chapman, 2001). We identified brain regions showing both shared and nonshared correlations with the dimensional scores. In particular, a number of brain regions showed GMVs in opposite directions of correlations between the two subscores and positive urgency. For instance, bilateral (but predominantly left‐hemispheric) insula GMVs were positively correlated with lack of premeditation and sensation seeking but negatively with positive urgency, in spite that lack of premeditation/sensation seeking and positive urgency traits were positively correlated. Thus, counter‐intuitively, higher GMVs of these shared regions are associated both with nonplanning and lack of forethought but with less vulnerability to emotion‐driven impulsivity. These shared volumetric correlates may reflect psychological processes diagonal between the UPPS‐P subdomains; that is, the positive urgency subscale captures not only behavioral traits distinct from lack of premeditation and sensation seeking but also those that are reflected in opposite directions in the regional GMVs. Psychometric research may help in refining the component processes of impulsivity and shedding light on what these opposing processes comprise.
4.4. Sex differences in the volumetric markers of dimensional impulsivity traits
Boys relative to girls showed higher impulsivity in all dimensions, consistent with the previous studies of a variety of different impulsivity measures in both children and adults (Cross, Copping, & Campbell, 2011; Pan et al., 2021; Van der Linden et al., 2006). Girls and boys also demonstrated differences in the volumetric correlates; for instance, boys vs. girls showed higher left anterior insula GMV in association with lack of premeditation; in contrast, girls vs. boys showed higher bilateral anterior insula GMVs in association with sensation seeking. Furthermore, we observed significantly stronger positive and negative correlations in girls relative to boys, as confirmed by slope tests, between sensation seeking and insula, putamen, and IFG GMVs and between positive urgency and cingulate cortex, insula, and IFG GMVs, respectively. Very few studies have examined sex differences in the neuroanatomical correlates of UPPS‐P traits. An earlier study of 152 adults with 2.5 times more women than men reported negative urgency in correlation with lower dmPFC and temporal pole GMVs but no evidence for sex differences in these correlations (Muhlert & Lawrence, 2015). In a study of behavioral activation/inhibition system (BAS/BIS) traits in young adults, females showed a negative correlation between BIS and parahippocampal gyral GMV as well as positive correlations between BAS and ventromedial PFC and inferior parietal lobule GMVs, whereas males showed the opposite pattern of correlations (Li et al., 2014). An ABCD study successfully classified the sex of 86% children based on morphometric analysis of image intensity values with the insula, precentral and postcentral gyri, and pericallosal sulcus as the most discernable features (Brennan, Wu, & Fan, 2020). Furthermore, these dimorphic features mediated the relationships between sex and clinical symptoms including depression, stress, and conduct problems. It remains to be seen whether the sex differences we observed here would manifest in clinical populations.
4.5. Heritability of UPPS‐P impulsivity and volumetric markers
We observed that in girls and boys combined the heritability for UPPS‐P impulsivity varied across dimensions, with lack of perseverance moderately (a 2 = 0.44) and the other four subdomains weakly heritable (a 2 = 0.21–0.28). Two recent studies of young adults showed moderate heritability for lack of perseverance (a 2 = 0.44, both) and negative urgency (a 2 = 0.40 and 0.35) and relatively low heritability for the other three UPPS‐P dimensions (a 2 = 0.27–0.32 and 0.18–0.27) (Friedman et al., 2020; Gustavson et al., 2019). Thus, genetic influences on lack of perseverance appear to be conserved through young adulthood. In contrast, other impulsivity traits, including negative urgency (a 2 = 0.40 from Friedman et al., 2020; 0.35 from Gustavson et al., 2019 vs. 0.22 from current sample), appear to show greater genetic influences in adults than in children, as with the heritability observed for general cognitive ability (Haworth et al., 2010) and cognitive control capacity (Chen et al., 2020). The heritability of sensation seeking seems varying widely across studies with a 2 = 0.28 in children (current sample) and (a 2 = 0.29 from Friedman et al., 2020; 0.18 from Gustavson et al., 2019) in adults, and 0.29–0.62 across a sample of 9,220 adolescents and young adults (Stoel, De Geus, & Boomsma, 2006).
The volumetric correlates of impulsivity showed much higher heritability than UPPS‐P subscores, consistent with our recent ABCD study on BAS/BIS traits (Ide et al., 2020). However, these findings contrast with studies of adults that showed comparable heritability in behavioral performance and volumetric correlates of fluid intelligence (Brouwer et al., 2014; Hulshoff Pol et al., 2006; Posthuma et al., 2002), working memory (Blokland et al., 2008; Karlsgodt et al., 2010), and risk‐taking (Rao, Zhou, Zheng, Yang, & Li, 2018). Thus, volumetric markers may serve as more reliable measures of the influence of genetics, as compared with clinical and behavioral assessments, on personality traits and cognitive performance in children but likely not in adults.
We observed significantly higher heritability for negative urgency in girls vs. boys and for lack of premeditation in boys vs. girls, suggesting potentially sex‐specific genetic contributions to dimensional impulsivity traits. Furthermore, the volumetric correlates of sensation seeking and positive urgency showed significantly higher heritability in girls than in boys, suggesting sex differences in genetic influences on how dimensional impulsivity manifests in cerebral volumetrics. On the other hand, behavioral genetic studies do not appear to be consistent with regard to sex differences in the heritability for externalizing psychopathologies (Saudino, Ronald, & Plomin, 2005). The current findings should thus be considered specific to developmentally typical children.
4.6. Limitations of the study, other considerations, and conclusions
A few limitations should be noted. Firstly, although the ABCD study comprises by far the largest sample of adolescents, we noted that the findings of girls and boys alone were statistically weaker than combined. This raises questions about the statistical power of the dataset in revealing sex‐specific volumetric correlates of impulsivity or sex differences in these correlates. Secondly, we presented cross‐sectional findings in the current study. Developmental brain maturation (Cho et al., 2013; Du et al., 2016) and environmental influences, such as intensive exploratory activities (Driemeyer, Boyke, Gaser, Buchel, & May, 2008; Schilling et al., 2013) may have significant influences on brain volumetrics. Future releases of the ABCD data would allow investigators to examine developmental and environmental impacts on UPPS‐P traits and their volumetric markers. Thirdly, in data analyses, we did not consider other clinical variables such as externalizing and internalizing behaviors, or clinical and subclinical conditions, such as depression, or medication status (Marmorstein, 2013). It remains to be seen how these variables may influence the current findings. Finally, we focused solely on the GMV; other morphometric measures such as cortical thickness and surface area may provide complementary measures of structural correlates of impulsive personality.
Studies have explored shared GMV correlates of impulsivity in neurotypical and clinical populations. For instance, GMV loss in the dmPFC and temporal pole has been linked to higher levels of negative urgency both in healthy young adults (Muhlert & Lawrence, 2015) and patients with cocaine use (Albein‐Urios et al., 2013) and gambling (Ruiz de Lara, Navas, Soriano‐Mas, Sescousse, & Perales, 2018) disorder, suggesting a shared structural deficit across externalizing traits and behaviors (Um, Whitt, Revilla, Hunton, & Cyders, 2019). However, the volumetric correlates of impulsivity did not consistently mirror between neurotypical and clinical populations (Pan et al., 2021). For instance, a VBM study showed that lack of premeditation was correlated positively and negatively with IFG and insula GMV, respectively, in adult cocaine users, whereas the reverse was observed in nondrug users (Moreno‐Lopez et al., 2012; Muller et al., 2015). Thus, again, the current findings should be considered specific to neurotypical children.
In conclusion, we demonstrated shared and nonshared volumetric markers of dimensional impulsivity traits, sex differences in these volumetric markers, as well as the heritability and sex differences in the heritability of impulsivity traits and their volumetric correlates. By elucidating the GM volumetric underpinnings of dimensional impulsivity, the current study may inform research of developmental psychology and potentially the etiologies of mental conditions that implicate impulsivity in children.
CONFLICT OF INTEREST
The authors declare no conflicts of interest in the current work.
ETHICS STATEMENT
The authors have obtained permission from the ABCD to use the Open and Restricted Access data for the current study. All recruitment procedures and informed consent forms, including consent to share de‐identified data, were approved by the Institutional Review Board of the University of California San Diego with study number 160091.
Supporting information
Appendix S1: Supporting Information
Chen, Y. , Ide, J. S. , Li, C. S. , Chaudhary, S. , Le, T. M. , Wang, W. , Zhornitsky, S. , Zhang, S. , & Li, C.‐S. R. (2022). Gray matter volumetric correlates of dimensional impulsivity traits in children: Sex differences and heritability. Human Brain Mapping, 43(8), 2634–2652. 10.1002/hbm.25810
Yu Chen and Jaime S. Ide contributed equally to this study.
Funding informationThis study was supported by NIH grant R01DA051922. The NIH is otherwise not responsible for the conceptualization of the study, data collection and analysis, or in the decision in publishing the results.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the ABCD project. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://abcdstudy.org/ with the permission of the ABCD study.
REFERENCES
- Albein‐Urios, N. , Martinez‐Gonzalez, J. M. , Lozano, O. , Moreno‐Lopez, L. , Soriano‐Mas, C. , & Verdejo‐Garcia, A. (2013). Negative urgency, disinhibition and reduced temporal pole gray matter characterize the comorbidity of cocaine dependence and personality disorders. Drug and Alcohol Dependence, 132, 231–237. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (DSM‐5®). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Anokhin, A. P. , Grant, J. D. , Mulligan, R. C. , & Heath, A. C. (2015). The genetics of impulsivity: Evidence for the heritability of delay discounting. Biological Psychiatry, 77, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou, E. , Um, M. , Wu, W. , & Cyders, M. A. (2020). Measurement invariance of the UPPS‐P impulsive behavior scale across age and sex across the adult life span. Assessment, 27, 432–453. [DOI] [PubMed] [Google Scholar]
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Barch, D. M. , Albaugh, M. D. , Avenevoli, S. , Chang, L. , Clark, D. B. , Glantz, M. D. , … Sher, K. J. (2017). Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Developmental Cognitive Neuroscience, 32, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt, E. S. , & Slaughter, L. (1998). Defining, measuring, and predicting impulsive aggression: A heuristic model. Behavioral Sciences & the Law, 16, 285–302. [DOI] [PubMed] [Google Scholar]
- Berg, J. M. , Latzman, R. D. , Bliwise, N. G. , & Lilienfeld, S. O. (2015). Parsing the heterogeneity of impulsivity: A meta‐analytic review of the behavioral implications of the UPPS for psychopathology. Psychological Assessment, 27, 1129–1146. [DOI] [PubMed] [Google Scholar]
- Bezdjian, S. , Baker, L. A. , & Tuvblad, C. (2011). Genetic and environmental influences on impulsivity: A meta‐analysis of twin, family and adoption studies. Clinical Psychology Review, 31, 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billieux, J. , Gay, P. , Rochat, L. , & Van der Linden, M. (2010). The role of urgency and its underlying psychological mechanisms in problematic behaviours. Behaviour Research and Therapy, 48, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Billieux, J. , Rochat, L. , Ceschi, G. , Carre, A. , Offerlin‐Meyer, I. , Defeldre, A. C. , … Van der Linden, M. (2012). Validation of a short French version of the UPPS‐P Impulsive Behavior Scale. Comprehensive Psychiatry, 53, 609–615. [DOI] [PubMed] [Google Scholar]
- Blokland, G. A. , McMahon, K. L. , Hoffman, J. , Zhu, G. , Meredith, M. , Martin, N. G. , … Wright, M. J. (2008). Quantifying the heritability of task‐related brain activation and performance during the N‐back working memory task: A twin fMRI study. Biological Psychology, 79, 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes, A. D. , Bechara, A. , Tranel, D. , Anderson, S. W. , Richman, L. , & Nopoulos, P. (2009). Right ventromedial prefrontal cortex: A neuroanatomical correlate of impulse control in boys. Social Cognitive and Affective Neuroscience, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, D. , Wu, T. & Fan, J. (2021). Morphometrical brain markers of sex difference. Cerebral Cortex, 31, 3641–3649. [DOI] [PubMed] [Google Scholar]
- Brouwer, R. M. , Hedman, A. M. , van Haren, N. E. , Schnack, H. G. , Brans, R. G. , Smit, D. J. , … Hulshoff Pol, H. E. (2014). Heritability of brain volume change and its relation to intelligence. NeuroImage, 100, 676–683. [DOI] [PubMed] [Google Scholar]
- Carlson, S. R. , Pritchard, A. A. , & Dominelli, R. M. (2013). Externalizing behavior, the UPPS‐P Impulsive Behavior scale and Reward and Punishment Sensitivity. Personality and Individual Differences, 54, 202–207. [Google Scholar]
- Casey, B. , Cannonier, T. , Conley, M. I. , Cohen, A. O. , Barch, D. M. , Heitzeg, M. M. , … Garavan, H. (2018). The adolescent brain cognitive development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain, S. R. , & Sahakian, B. J. (2007). The neuropsychiatry of impulsivity. Current Opinion in Psychiatry, 20, 255–261. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Chaudhary, S. , Wang, W. , & Li, C.‐S. R. (2022). Gray matter volumes of the insula and anterior cingulate cortex and their dysfunctional roles in cigarette smoking. Addiction Neuroscience, 1, 100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Chen, C. , Wu, T. , Qiu, B. , Zhang, W. , & Fan, J. (2020). Accessing the development and heritability of the capacity of cognitive control. Neuropsychologia, 139, 107361. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Li, G. , Ide, J. S. , Luo, X. , & Li, C.‐S. R. (2021). Sex differences in attention deficit hyperactivity symptom severity and functional connectivity of the dorsal striatum in young adults. Neuroimage: Reports, 1, 100025. [Google Scholar]
- Cho, S. S. , Pellecchia, G. , Aminian, K. , Ray, N. , Segura, B. , Obeso, I. , & Strafella, A. P. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Cambridge, MA: Academic Press. [Google Scholar]
- Conners, C. K. , Staff, M. , Connelly, V. , Campbell, S. , MacLean, M. , & Barnes, J. (2000). Conners' continuous performance test II (CPT II v. 5) (Vol. 29, pp. 175–196). San Antonio, TX: North Tonawanda. [Google Scholar]
- Cross, C. P. , Copping, L. T. , & Campbell, A. (2011). Sex differences in impulsivity: A meta‐analysis. Psychological Bulletin, 137, 97–130. [DOI] [PubMed] [Google Scholar]
- Cyders, M. A. (2013). Impulsivity and the sexes: Measurement and structural invariance of the UPPS‐P Impulsive Behavior Scale. Assessment, 20, 86–97. [DOI] [PubMed] [Google Scholar]
- Cyders, M. A. , Littlefield, A. K. , Coffey, S. , & Karyadi, K. A. (2014). Examination of a short English version of the UPPS‐P Impulsive Behavior Scale. Addictive Behaviors, 39, 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders, M. A. , & Smith, G. T. (2008). Emotion‐based dispositions to rash action: Positive and negative urgency. Psychological Bulletin, 134, 807–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders, M. A. , Smith, G. T. , Spillane, N. S. , Fischer, S. , Annus, A. M. , & Peterson, C. (2007). Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychological Assessment, 19, 107–118. [DOI] [PubMed] [Google Scholar]
- Dekker, M. R. , & Johnson, S. L. (2018). Major depressive disorder and emotion‐related impulsivity: Are both related to cognitive inhibition? Cognitive Therapy and Research, 42, 398–407. [Google Scholar]
- Dhingra, I. , Zhang, S. , Zhornitsky, S. , Le, T. M. , Wang, W. , Chao, H. H. , … Li, C.‐S. R. (2020). The effects of age on reward magnitude processing in the monetary incentive delay task. NeuroImage, 207, 116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer, J. , Boyke, J. , Gaser, C. , Buchel, C. , & May, A. (2008). Changes in gray matter induced by learning—Revisited. PLoS One, 3, e2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X. , Qi, X. , Yang, Y. , Du, G. , Gao, P. , Zhang, Y. , … Zhang, Q. (2016). Altered structural correlates of impulsivity in adolescents with internet gaming disorder. Frontiers in Human Neuroscience, 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme, S. , Hudziak, J. J. , Botteron, K. N. , Ganjavi, H. , Lepage, C. , Collins, D. L. , … Brain Development Cooperative Group . (2011). Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biological Psychiatry, 70, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, N. P. , Hatoum, A. S. , Gustavson, D. E. , Corley, R. P. , Hewitt, J. K. , & Young, S. E. (2020). Executive functions and impulsivity are genetically distinct and independently predict psychopathology: Results from two adult twin studies. Clinical Psychological Science, 8, 519–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz, C. O. , Morris, P. E. , & Richler, J. J. (2012). Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General, 141, 2–18. [DOI] [PubMed] [Google Scholar]
- Gaser, C. & Dahnke, R. (2016). Organization for Human Brain Mapping.
- Grigg‐Damberger, M. , & Ralls, F. (2013). Treatment strategies for complex behavioral insomnia in children with neurodevelopmental disorders. Current Opinion in Pulmonary Medicine, 19, 616–625. [DOI] [PubMed] [Google Scholar]
- Gustavson, D. E. , Franz, C. E. , Kremen, W. S. , Carver, C. S. , Corley, R. P. , Hewitt, J. K. , & Friedman, N. P. (2019). Common genetic influences on impulsivity facets are related to goal management, psychopathology, and personality. Journal of Research in Personality, 79, 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth, C. M. , Wright, M. J. , Luciano, M. , Martin, N. G. , de Geus, E. J. , van Beijsterveldt, C. E. , … Davis, O. S. (2010). The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry, 15, 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, B. M. , Blonigen, D. M. , Kramer, M. D. , Krueger, R. F. , Patrick, C. J. , Iacono, W. G. , & McGue, M. (2007). Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology, 116, 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol, H. E. , Schnack, H. G. , Posthuma, D. , Mandl, R. C. , Baare, W. F. , van Oel, C. , … Kahn, R. S. (2006). Genetic contributions to human brain morphology and intelligence. The Journal of Neuroscience, 26, 10235–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide, J. S. , Li, H. T. , Chen, Y. , Le, T. M. , Li, C. S. P. , Zhornitsky, S. , & Li, C. R. (2020). Gray matter volumetric correlates of behavioral activation and inhibition system traits in children: An exploratory voxel‐based morphometry study of the ABCD project data. NeuroImage, 220, 117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide, J. S. , Tung, H. C. , Yang, C.‐T. , Tseng, Y.‐C. , & Li, C.‐S. R. (2017). Barratt impulsivity in healthy adults is associated with higher Gray matter concentration in the parietal occipital cortex that represents peripheral visual field. Frontiers in Human Neuroscience, 11, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S. L. , Carver, C. S. , & Joormann, J. (2013). Impulsive responses to emotion as a transdiagnostic vulnerability to internalizing and externalizing symptoms. Journal of Affective Disorders, 150, 872–878. [DOI] [PubMed] [Google Scholar]
- Johnson, S. L. , Elliott, M. V. , & Carver, C. S. (2020). Impulsive responses to positive and negative emotions: Parallel neurocognitive correlates and their implications. Biological Psychiatry, 87, 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S. L. , Tharp, J. A. , Peckham, A. D. , Sanchez, A. H. , & Carver, C. S. (2016). Positive urgency is related to difficulty inhibiting prepotent responses. Emotion, 16, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt, K. H. , Kochunov, P. , Winkler, A. M. , Laird, A. R. , Almasy, L. , Duggirala, R. , … Glahn, D. C. (2010). A multimodal assessment of the genetic control over working memory. Journal of Neuroscience, 30, 8197–8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby, K. N. , Petry, N. M. , & Bickel, W. K. (1999). Heroin addicts have higher discount rates for delayed rewards than non‐drug‐using controls. Journal of Experimental Psychology: General, 128, 78–87. [DOI] [PubMed] [Google Scholar]
- Kleber, B. , Veit, R. , Moll, C. V. , Gaser, C. , Birbaumer, N. , & Lotze, M. (2016). Voxel‐based morphometry in opera singers: Increased gray‐matter volume in right somatosensory and auditory cortices. NeuroImage, 133, 477–483. [DOI] [PubMed] [Google Scholar]
- Kohler, H.‐P. , Behrman, J. R. , & Schnittker, J. (2011). Social science methods for twins data: Integrating causality, endowments, and heritability. Biodemography and Social Biology, 57, 88–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korponay, C. , Dentico, D. , Kral, T. , Ly, M. , Kruis, A. , Goldman, R. , … Davidson, R. J. (2017). Neurobiological correlates of impulsivity in healthy adults: Lower prefrontal gray matter volume and spontaneous eye‐blink rate but greater resting‐state functional connectivity in basal ganglia‐thalamo‐cortical circuitry. NeuroImage, 157, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek, M. J. , Nielsen, D. A. , Butelman, E. R. , & LaForge, K. S. (2005). Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience, 8, 1450–1457. [DOI] [PubMed] [Google Scholar]
- Le, T. M. , Wang, W. , Zhornitsky, S. , Dhingra, I. , Zhang, S. , & Li, C.‐S. R. (2019). Reward sensitivity and electrodermal responses to actions and outcomes in a go/no‐go task. PLoS One, 14, e0219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Chen, Y. , Le, T. M. , Wang, W. , Tang, X. , & Li, C. R. (2021). Neural correlates of individual variation in two‐back working memory and the relationship with fluid intelligence. Scientific Reports, 11, 9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Chen, Y. , Wang, W. , Dhingra, I. , Zhornitsky, S. , Tang, X. , & Li, C. R. (2020). Sex differences in neural responses to the perception of social interactions. Frontiers in Human Neuroscience, 14, 565132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Qiao, L. , Sun, J. , Wei, D. , Li, W. , Qiu, J. , … Shi, H. (2014). Gender‐specific neuroanatomical basis of behavioral inhibition/approach systems (BIS/BAS) in a large sample of young adults: A voxel‐based morphometric investigation. Behavioural Brain Research, 274, 400–408. [DOI] [PubMed] [Google Scholar]
- Mackey, S. , Chaarani, B. , Kan, K. J. , Spechler, P. A. , Orr, C. , Banaschewski, T. , … IMAGEN Consortium . (2017). Brain regions related to impulsivity mediate the effects of early adversity on antisocial behavior. Biological Psychiatry, 82, 275–282. [DOI] [PubMed] [Google Scholar]
- Manjon, J. V. , Coupe, P. , Marti‐Bonmati, L. , Collins, D. L. , & Robles, M. (2010). Adaptive non‐local means denoising of MR images with spatially varying noise levels. Journal of Magnetic Resonance Imaging, 31, 192–203. [DOI] [PubMed] [Google Scholar]
- Marmorstein, N. R. (2013). Associations between dispositions to rash action and internalizing and externalizing symptoms in children. Journal of Clinical Child and Adolescent Psychology, 42, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta, A.d. , Gonçalves, F. L. , & Bizarro, L. (2012). Delay discounting: Concepts and measures. Psychology & Neuroscience, 5, 135–146. [Google Scholar]
- Merz, E. C. , He, X. , & Noble, K. G. (2018). Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage: Clinical, 20, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. A. , & Chapman, J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. [DOI] [PubMed] [Google Scholar]
- Miller, J. , Flory, K. , Lynam, D. , & Leukefeld, C. (2003). A test of the four‐factor model of impulsivity‐related traits. Personality and Individual Differences, 34, 1403–1418. [Google Scholar]
- Moeller, F. G. , Barratt, E. S. , Dougherty, D. M. , Schmitz, J. M. , & Swann, A. C. (2001). Psychiatric aspects of impulsivity. American Journal of Psychiatry, 158, 1783–1793. [DOI] [PubMed] [Google Scholar]
- Moreno‐Lopez, L. , Catena, A. , Fernandez‐Serrano, M. J. , Delgado‐Rico, E. , Stamatakis, E. A. , Perez‐Garcia, M. , & Verdejo‐Garcia, A. (2012). Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence, 125, 208–214. [DOI] [PubMed] [Google Scholar]
- Muhlert, N. , & Lawrence, A. D. (2015). Brain structure correlates of emotion‐based rash impulsivity. NeuroImage, 115, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, V. I. , Langner, R. , Cieslik, E. C. , Rottschy, C. , & Eickhoff, S. B. (2015). Interindividual differences in cognitive flexibility: Influence of gray matter volume, functional connectivity and trait impulsivity. Brain Structure & Function, 220, 2401–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen, L. K. , & Muthen, B. O. (2012). Mplus user's guide (7th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nichols, T. E. (2012). Multiple testing corrections, nonparametric methods, and random field theory. NeuroImage, 62, 811–815. [DOI] [PubMed] [Google Scholar]
- Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin, 126, 220–2461. [DOI] [PubMed] [Google Scholar]
- Niv, S. , Tuvblad, C. , Raine, A. , Wang, P. , & Baker, L. A. (2012). Heritability and longitudinal stability of impulsivity in adolescence. Behavior Genetics, 42, 378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum, A. L. (2011). Delay discounting: I'm ak, you're ak. Journal of the Experimental Analysis of Behavior, 96, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens, M. M. , Hyatt, C. S. , Gray, J. C. , Miller, J. D. , Lynam, D. R. , Hahn, S. , … Garavan, H. (2020). Neuroanatomical correlates of impulsive traits in children aged 9 to 10. Journal of Abnormal Psychology, 129, 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, N. , Wang, S. , Zhao, Y. , Lai, H. , Qin, K. , Li, J. , … Gong, Q. (2021). Brain gray matter structures associated with trait impulsivity: A systematic review and voxel‐based meta‐analysis. Human Brain Mapping, 42, 2214–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, J. H. , Stanford, M. S. , & Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Paus, T. , Keshavan, M. , & Giedd, J. N. (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeia, S. , Inacio, L. M. , de Freitas, R. S. , Zanini, G. V. , Malloy‐Diniz, L. , & Cogo‐Moreira, H. (2018). Psychometric properties of a short version of the impulsiveness questionnaire UPPS‐P in a Brazilian adult sample: Invariance for effects of age, sex and socioeconomic status and subscales viability. Frontiers in Psychology, 9, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma, D. , De Geus, E. J. , Baaré, W. F. , Pol, H. E. H. , Kahn, R. S. , & Boomsma, D. I. (2002). The association between brain volume and intelligence is of genetic origin. Nature Neuroscience, 5, 83–84. [DOI] [PubMed] [Google Scholar]
- Rajapakse, J. C. , Giedd, J. N. , & Rapoport, J. L. (1997). Statistical approach to segmentation of single‐channel cerebral MR images. IEEE Transactions on Medical Imaging, 16, 176–186. [DOI] [PubMed] [Google Scholar]
- Rao, L.‐L. , Zhou, Y. , Zheng, D. , Yang, L.‐Q. , & Li, S. (2018). Genetic contribution to variation in risk taking: A functional MRI twin study of the balloon analogue risk task. Psychological Science, 29, 1679–1691. [DOI] [PubMed] [Google Scholar]
- Reynolds, B. , & Schiffbauer, R. (2004). Measuring state changes in human delay discounting: An experiential discounting task. Behavioural Processes, 67, 343–356. [DOI] [PubMed] [Google Scholar]
- Rijsdijk, F. V. , & Sham, P. C. (2002). Analytic approaches to twin data using structural equation models. Briefings in Bioinformatics, 3, 119–133. [DOI] [PubMed] [Google Scholar]
- Ruiz de Lara, C. M. , Navas, J. F. , Soriano‐Mas, C. , Sescousse, G. , & Perales, J. C. (2018). Regional grey matter volume correlates of gambling disorder, gambling‐related cognitive distortions, and emotion‐driven impulsivity. International Gambling Studies, 18, 195–216. [Google Scholar]
- Saudino, K. J. , Ronald, A. , & Plomin, R. (2005). The etiology of behavior problems in 7‐year‐old twins: Substantial genetic influence and negligible shared environmental influence for parent ratings and ratings by same and different teachers. Journal of Abnormal Child Psychology, 33, 113–130. [DOI] [PubMed] [Google Scholar]
- Schilling, C. , Kuhn, S. , Romanowski, A. , Banaschewski, T. , Barbot, A. , Barker, G. J. , … IMAGEN Consortium . (2013). Common structural correlates of trait impulsiveness and perceptual reasoning in adolescence. Human Brain Mapping, 34, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby, E. A. , Anestis, M. D. , & Joiner, T. E. (2008). Understanding the relationship between emotional and behavioral dysregulation: Emotional cascades. Behaviour Research and Therapy, 46, 593–611. [DOI] [PubMed] [Google Scholar]
- Stanford, M. S. , Mathias, C. W. , Dougherty, D. M. , Lake, S. L. , Anderson, N. E. , & Patton, J. H. (2009). Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences, 47, 385–395. [Google Scholar]
- Stoel, R. D. , De Geus, E. J. , & Boomsma, D. I. (2006). Genetic analysis of sensation seeking with an extended twin design. Behavior Genetics, 36, 229–237. [DOI] [PubMed] [Google Scholar]
- Tervo‐Clemmens, B. , Quach, A. , Luna, B. , Foran, W. , Chung, T. , De Bellis, M. D. , & Clark, D. B. (2017). Neural correlates of rewarded response inhibition in youth at risk for problematic alcohol use. Frontiers in Behavioral Neuroscience, 11, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiego, J. , Chamberlain, S. R. , Harrison, B. J. , Dawson, A. , Albertella, L. , Youssef, G. J. , … Yücel, M. (2020). Heritability of overlapping impulsivity and compulsivity dimensional phenotypes. Scientific Reports, 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernegg, M. , Pletzer, B. , Schwartenbeck, P. , Ludersdorfer, P. , Hoffmann, U. , & Kronbichler, M. (2015). Impulsivity relates to striatal gray matter volumes in humans: Evidence from a delay discounting paradigm. Frontiers in Human Neuroscience, 9, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um, M. , Whitt, Z. T. , Revilla, R. , Hunton, T. , & Cyders, M. A. (2019). Shared neural correlates underlying addictive disorders and negative urgency. Brain Sciences, 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden, M. , d'Acremont, M. , Zermatten, A. , Jermann, F. , Larøi, F. , Willems, S. , … Bechara, A. (2006). A French adaptation of the UPPS Impulsive Behavior Scale. European Journal of Psychological Assessment, 22, 38–42. [Google Scholar]
- Visscher, P. M. , Hill, W. G. , & Wray, N. R. (2008). Heritability in the genomics era—Concepts and misconceptions. Nature Reviews Genetics, 9, 255–266. [DOI] [PubMed] [Google Scholar]
- Visscher, P. M. , Medland, S. E. , Ferreira, M. A. , Morley, K. I. , Zhu, G. , Cornes, B. K. , … Martin, N. G. (2006). Assumption‐free estimation of heritability from genome‐wide identity‐by‐descent sharing between full siblings. PLoS Genetics, 2, e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer, J. , Baggott, M. J. , & de Wit, H. (2013). Test–retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Experimental and Clinical Psychopharmacology, 21, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, A. , & Dannon, P. (2015). Is impulsivity a male trait rather than female trait? Exploring the sex difference in impulsivity. Current Behavioral Neuroscience Reports, 2, 9–14. [Google Scholar]
- Whiteside, S. P. , Lynam, D. R. , Miller, J. D. , & Reynolds, S. K. (2005). Validation of the UPPS impulsive behaviour scale: A four‐factor model of impulsivity. European Journal of Personality, 19, 559–574. [Google Scholar]
- Wilke, M. , Holland, S. K. , Altaye, M. , & Gaser, C. (2008). Template‐O‐Matic: A toolbox for creating customized pediatric templates. NeuroImage, 41, 903–913. [DOI] [PubMed] [Google Scholar]
- Woo, C. W. , Krishnan, A. , & Wager, T. D. (2014). Cluster‐extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski, T. C. , Cyders, M. A. , & Smith, G. T. (2009). Positive urgency predicts illegal drug use and risky sexual behavior. Psychology of Addictive Behaviors, 23, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski, T. C. , Stairs, A. M. , Settles, R. F. , Combs, J. L. , & Smith, G. T. (2010). The measurement of dispositions to rash action in children. Assessment, 17, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar, J. H. (1999). Biostatistical analysis. London, England: Pearson Education India. [Google Scholar]
- Zucker, R. A. (2008). Anticipating problem alcohol use developmentally from childhood into middle adulthood: What have we learned? Addiction, 103, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the ABCD project. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://abcdstudy.org/ with the permission of the ABCD study.


