Multicomponent reaction for the synthesis of tri-substituted 4H-pyrans and tetra-substituted pyranopyrazoles.
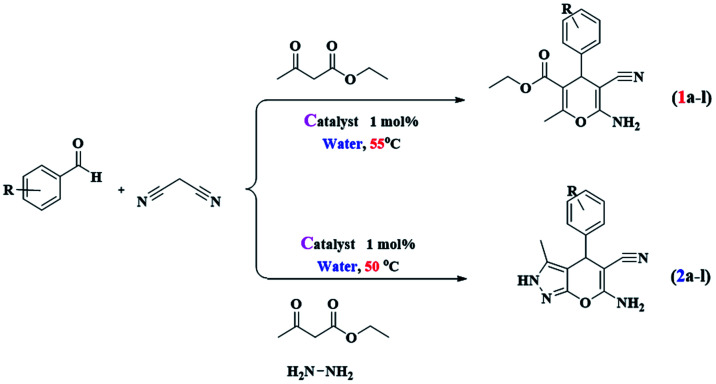
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Aldehyde | Product | Time (min) | Yielda (%) | TON | TOF | Mp (°C) | Ref. | |
| Obtained | Reported | ||||||||
| 1 | Benzaldehyde | 1a | 15 | 97 | 107.7 | 430 | 209–210 | 208–210 | 51 |
| 2 | 4-Chlorobenzaldehyde | 1b | 15 | 93 | 103.3 | 413.2 | 172 | 172–174 | 51 |
| 3 | 4-Bromobenzaldehyde | 1c | 10 | 92 | 102.2 | 638.8 | 178–179 | 178 | 52 |
| 4 | 4-Hydroxybenzaldehyde | 1d | 15 | 90 | 100 | 400 | 177 | 175–177 | 53 |
| 5 | 4-Methoxybenzaldehyde | 1e | 20 | 95 | 105.5 | 319.8 | 137 | 135–137 | 52 |
| 6 | 4-Methylbenzaldehyde | 1f | 20 | 85 | 94.4 | 286.2 | 174–176 | 175–177 | 51 |
| 7 | 4-Fluorobenzaldehyde | 1g | 10 | 94 | 104.4 | 652.7 | 274–276 | 274–277 | 51 |
| 8 | 4-Nitrobenzaldehyde | 1h | 15 | 88 | 97.7 | 391.1 | 182–183 | 180–183 | 54 |
| 9 | 3-Nitrobenzaldehyde | 1i | 15 | 83 | 92.2 | 368.8 | 190 | 189–191 | 55 |
| 10 | 2-Nitrobenzaldehyde | 1j | 15 | 90 | 100 | 400 | 181–182 | 181–183 | 56 |
| 11 | 2-Chlorobenzaldehyde | 1k | 10 | 95 | 105.5 | 659.7 | 194–195 | 193–195 | 55 |
| 12 | 2-Bromobenzaldehyde | 1l | 15 | 95 | 105.5 | 422.2 | 186 | 185–187 | 55 |
| 13 | Benzaldehyde | 2a | 15 | 98 | 108.8 | 435.5 | 262–263 | 261–263 | 57 |
| 14 | 4-Chlorobenzaldehyde | 2b | 15 | 95 | 105.5 | 422.2 | 231–233 | 231 | 57 |
| 15 | 4-Bromobenzaldehyde | 2c | 15 | 90 | 100 | 400 | 172–174 | 172–174 | 58 |
| 16 | 4-Hydroxybenzaldehyde | 2d | 20 | 90 | 100 | 303 | 254–256 | 255–257 | 59 |
| 17 | 4-Methoxybenzaldehyde | 2e | 20 | 88 | 97.7 | 296.3 | 211–212 | 210–212 | 60 |
| 18 | 4-Methylbenzaldehyde | 2f | 25 | 88 | 97.7 | 234 | 213–215 | 212–215 | 61 |
| 19 | 4-Fluorobenzaldehyde | 2g | 15 | 90 | 100 | 400 | 239–241 | 240–242 | 62 |
| 20 | 4-Nitrobenzaldehyde | 2h | 20 | 95 | 105.5 | 319.8 | 247–249 | 246–248 | 60 |
| 21 | 3-Nitrobenzaldehyde | 2i | 20 | 92 | 102.2 | 309.7 | 194 | 192–194 | 60 |
| 22 | 2-Nitrobenzaldehyde | 2j | 20 | 95 | 105.5 | 319.8 | 216 | 216–218 | 57 |
| 23 | 2-Chlorobenzaldehyde | 2k | 20 | 85 | 94.4 | 286.2 | 241–243 | 240–242 | 60 |
| 24 | 2-Bromobenzaldehyde | 2l | 20 | 83 | 92.2 | 279.5 | 232–233 | 232–235 | 61 |
Isolated yield.
