Figure 1:
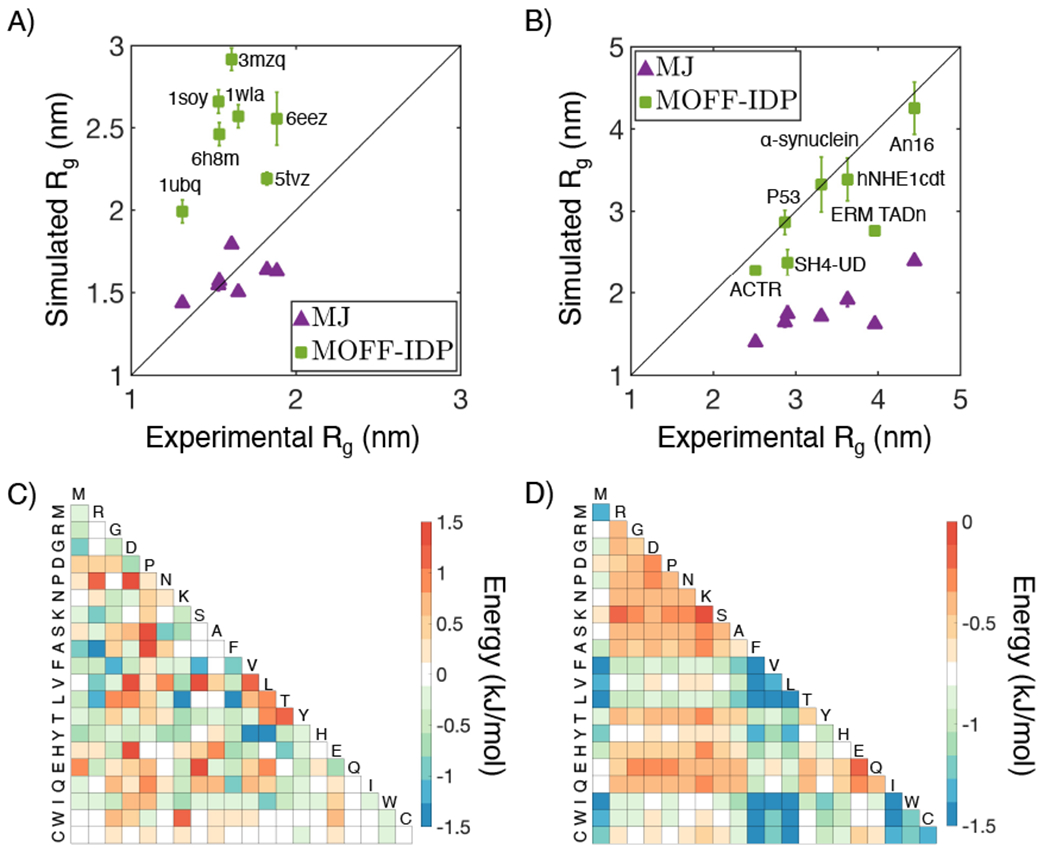
Existing coarse-grained protein force fields are often limited to either folded or disordered proteins, but not both. For example, MJ performs well on folded (A) but not disordered (B) proteins. The opposite trend can be seen for MOFF-IDP, an IDP force field introduced in Ref. [36]. The interaction matrices between amino acids for the two force fields are shown in parts C (MOFF-IDP) and D (MJ). The energy function for MJ can be found in Ref. [39], and interactions among amino acids are based on a scaled Miyazawa-Jernigan (MJ) potential by a factor of 0.6.
