Abstract
OBJECTIVES
The aim of this trial was to determine whether ultrasound-assisted thrombolysis (USAT) is superior to standard catheter-directed thrombolysis (SCDT) in pulmonary arterial thrombus reduction for patients with submassive pulmonary embolism (sPE).
BACKGROUND
Catheter-directed therapy has been increasingly used in sPE and massive pulmonary embolism as a decompensation prevention and potentially lifesaving procedure. It is unproved whether USAT is superior to SCDT using traditional multiple-side-hole catheters in the treatment of patients with pulmonary embolism.
METHODS
Adults with sPE were enrolled. Participants were randomized 1:1 to USAT or SCDT. The primary outcome was 48-hour clearance of pulmonary thrombus assessed by pre- and postprocedural computed tomographic angiography using a refined Miller score. Secondary outcomes included improvement in right ventricular–to–left ventricular ratio, intensive care unit and hospital stay, bleeding, and adverse events up to 90 days.
RESULTS
Eighty-one patients with acute sPE were randomized and were available for analysis. The mean total dose of alteplase for USAT was 19 ± 7 mg and for SCDT was 18 ± 7 mg (P = 0.53), infused over 14 ± 6 and 14 ± 5 hours, respectively (P = 0.99). In the USAT group, the mean raw pulmonary arterial thrombus score was reduced from 31 ± 4 at baseline to 22 ± 7 (P < 0.001). In the SCDT group, the score was reduced from 33 ± 4 to 23 ± 7 (P < 0.001). There was no significant difference in mean thrombus score reduction between the 2 groups (P = 0.76). The mean reduction in right ventricular/left ventricular ratio from baseline (1.54 ± 0.30 for USAT, 1.69 ± 0.44 for SCDT) to 48 hours was 0.37 ± 0.34 in the USAT group and 0.59 ± 0.42 in the SCDT group (P = 0.01). Major bleeding (1 stroke and 1 vaginal bleed requiring transfusion) occurred in 2 patients, both in the USAT group.
CONCLUSIONS
In the SUNSET sPE (Standard vs. Ultrasound-Assisted Catheter Thrombolysis for Submassive Pulmonary Embolism) trial, patients undergoing USAT had similar pulmonary arterial thrombus reduction compared with those undergoing SCDT, using comparable mean lytic doses and durations of lysis.
Keywords: catheter thrombolysis, EKOS, pulmonary embolism, pulmonary hypertension, ultrasound-assisted thrombolysis
Catheter-directed thrombolysis (CDT) has been increasingly used in both submassive (intermediate-risk) and massive (high-risk) pulmonary embolism (PE) as a decompensation prevention and potentiallly lifesaving procedure (1–3). It has particularly peaked over the past few years after a meta-analysis of large randomized trials confirmed the mortality benefit with the use of systemic thrombolysis (4). Given that this benefit came at the cost of major bleeding complications, CDT emerged as a potentially safer and broader indicated alternative, as it uses only a fraction of the systemic lytic dose, given over a longer time frame (1–5).
CDT in all vascular beds, including the pulmonary arteries (PAs), has been traditionally delivered through standard multiple-side-hole catheters placed within the clot, slowly infusing lytic agent for its dissolution. On the basis of in vitro studies indicating ultrasound waves as enhancers of thrombolytic penetration and clot clearance, the concept of ultrasound-assisted thrombolysis (USAT) is intriguing (6,7). This led to the development of the EkoSonic Endovascular System (EKOS), which uses an intracatheter ultrasonic core to deliver acoustic energy in combination with lytic pharmacotherapy at the site of clot burden. The purported clinical benefit of the EKOS catheter is that similar thrombus clearance may be achieved using lower doses of lytic agents and/or shorter duration of therapy, thus potentially reducing complication rates and hospital stay. The EKOS system was, until recently, the only U.S. Food and Drug Administration–approved lytic catheter for PE, resulting in preferential usage in CDT practice (5,8–11).
However, it is unproved whether USAT using the EKOS catheter is superior to standard (non-ultrasound-assisted) CDT using traditional multiple-side-hole catheters in the treatment of patients with PE, with existing studies being controversial (5,12–18). In an era of increasing focus on quality and cost consciousness, the preferential use of USAT over SCDT should be rigorously studied. To address the comparative effectiveness between these 2 treatment options, we performed the SUNSET sPE (Standard vs. Ultrasound-Assisted Catheter Thrombolysis for Submassive Pulmonary Embolism) trial to measure differences in pulmonary arterial thrombus reduction.
METHODS
TRIAL ORGANIZATION.
SUNSET sPE is a multi-center, randomized, head-to-head, single-blind clinical trial (NCT02758574). All 3 enrolling centers supported the trial with internal funding. The American Venous Forum Foundation provided additional funding through the JOBST award primarily to support a biomarker trial among patients with PE. No industry funding has been obtained for the conduct of the research, and the study investigators have no relevant financial interests in the outcome of the study.
The trial was approved by the Institutional Review Boards at all participating centers. The steering committee and site investigators were responsible for the design and conduct of the trial, respectively (19). The steering committee vouches for the accuracy and completeness of the data and the analyses and for the fidelity of the trial to the protocol.
PATIENT POPULATION.
Patients with acute intermediate-risk (submassive) PE were enrolled at 3 clinical centers in the United States. Patients were recruited at the emergency departments or intensive care units (ICUs) of the participating centers, and written informed consent was obtained before randomization. Eligibility was considered for those diagnosed with submassive PE (sPE) and planned to undergo CDT using an algorithm consistent with the PERT Consortium treatment algorithm (20). Diagnosis of sPE was defined as the combination of: 1) PE diagnosed by computed tomography angiography (CTA); 2) right ventricular (RV) strain as diagnosed by RV–to–left ventricular(LV) diameter ratio >1 by either CTA or transthoracic echocardiography and/or elevated cardiac biomarkers (either troponin or brain-natriuretic peptide); and 3) absence of circulatory shock as defined by cardiac arrest, persistent hypotension (systolic blood pressure <90 mm Hg), or requirement for vasoactive medications. Patients were excluded for age <18 years, symptoms for >14 days, elevated bleeding risk (any prior intracranial hemorrhage, known structural intracranial cerebrovascular disease or neoplasm, ischemic stroke within 3 months, suspected aortic dissection, active bleeding or bleeding diathesis, recent spinal or cranial/brain surgery, and recent closed-head or facial trauma with bony fracture or brain injury), participation in any other investigational drug or device study, life expectancy <90 days, or inability to comply with study assessments.
RANDOMIZATION.
Patients were randomized independently at each of the 3 centers using a permuted block design, ensuring 1:1 balance in USAT versus CDT treatment arm allocation over time for each center. The randomization sequence was created by each center’s statistician with a user-written Stata command: ralloc. Thereafter, treatment allocation was placed in a sequentially numbered, sealed, and opaque envelope accessible only to the study coordinator and principal investigators.
PROCEDURES.
Patients underwent baseline CTA, transthoracic echocardiography, and cardiac biomarker assessment, including troponin I and brain natriuretic peptide levels. Baseline vital signs, including heart rate, pulse oximetry, and oxygen requirement at rest, were documented. If the patient was an appropriate candidate for CDT, the trial was discussed and informed consent was obtained.
All participants were taken to the interventional suite to undergo the study procedure, which involved positioning of 1 (for unilateral PE) or 2 pulmonary arterial infusion catheters, 1 into each main PA, under fluoroscopic guidance via percutaneous transvenous access. The specific catheters used differed by intervention arm: the experimental arm received the EKOS USAT catheter (6- or 12-cm infusion length), and the control arm received a standard Cragg-McNamara (Medtronic) or Uni-Fuse (AngioDynamics) multiple-side-hole catheter (5- or 10-cm infusion length). Invasive PA systolic and diastolic pressures were transduced and documented during the procedure. Alteplase, a recombinant tissue plasminogen activator (tPA), was the drug infused in all patients.
Technical details of the procedure, including choice of access site, concomitant inferior vena cava filter placement, and intraoperative and postoperative tPA dosing, were left to the discretion of the treating physician; however, study protocol recommended that the maximum tPA dosing should not exceed 24 mg. In May 2017, preliminary results from the OPTALYSE PE (Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Pulmonary Embolism) study, in which 4 different tPA dosing and duration protocols in USAT were assessed, showed that shorter periods of thrombolysis and smaller tPA doses may be sufficient to achieve thrombolysis (10). On the basis of these findings, we amended the study protocol to include a recommendation to treating physicians that 4 to 8 mg of tPA should be given through each catheter over a 4- to 8-hour period, with no loading dose. Termination was considered at this point, provided that the patient’s hemodynamic or respiratory parameters had improved. This recommendation applied to both the SCDT and USAT arms of the study.
All patients remained in the ICU while tPA was administered. Heparin was administered concomitantly, with doses determined using a hospitaldefined nomogram that protocolizes dosing to target a low therapeutic anti-Xa level or partial thromboplastin time (60 seconds). Prior to catheter removal PA pressures were transduced. Infusion catheters were removed at the bedside upon improvement of hemodynamic and clinical parameters, without follow-up pulmonary angiography. Pulmonary CTA and echocardiography were scheduled within 48 ± 6 hours.
Postlysis care followed standard institutional algorithms for patients with PE. Anticoagulation was continued after discharge in all patients. Bleeding risk, insurance coverage, anticoagulation history, patient preference, and other factors were considered, and the choice of specific agent (which could include warfarin, enoxaparin, or a direct-acting oral anticoagulant agent) was individualized to specific patient needs.
Two follow-up visits were planned, at 3 months and 12 months after the study procedure. All patients were asked to return to the PE response team follow-up clinic.
OUTCOMES.
The primary outcome, thrombus load reduction, was measured by the change in the computed tomographic (CT) obstruction score using the refined modified Miller scoring system. Scoring was performed by 2 independent reviewers blinded to the treatment arm (the study was single blind, as patients were aware of the treatment they were offered). Data were compared to assess interobserver agreement. The reviewers were cardiothoracictrained radiologists with 3 years (J.S.) and 25 years (J.L.) of postresidency experience in interpreting CTA of the PAs. The score was measured by dividing the pulmonary arterial tree into 10 segmental arteries per lung (3 for the upper lobes, 2 for the middle lobe or lingula, and 5 for the lower lobes) and assessing thrombus burden by a weighing combination of thrombus presence and percentage of obstruction. Although some patients had the standard 10 segments per lung, some had more or less, so total segments were amended to reflect true PA anatomy. Percentage obstruction was then calculated on the basis of total segments to yield the final CT obstruction index (Supplemental Appendix). The CT obstruction score has been previously described and validated as a quantifiable measure of pulmonary clot burden (21,22). The thrombus load reduction was recorded as the difference in the CT obstruction score on the basis of repeat CTA obtained within 48 ± 6 hours post-CDT compared with pretreatment baseline CTA.
A secondary efficacy endpoint was change in RV/LV diameter ratio as measured by CTA from baseline to 48 ± 6 hours after the end of lytic infusion. Ventricular measurements were made on axial images and obtained by measuring the maximum width of the ventricle from compacted to compacted myocardium in a plane perpendicular to the long axis of the ventricle. Measurements were done by the same 2 blinded reviewers who scored the primary outcome. Interobserver agreement between radiologists for thrombus scoring and RV/LV ratio measurements was calculated.
Other secondary endpoints included ICU and hospital stay, death, hemodynamic decompensation as defined in inclusion criterion 3, major and minor bleeding, recurrent venous thromboembolism, and serious adverse events up to 90 days after randomization. Major bleeding was defined as overt bleeding associated with a fall in hemoglobin level of at least2.0 g/dL or with transfusion of $2 U of red blood cells or involvement of a critical site (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome) or need for operating room intervention. Clinically overt bleeding not fulfilling the criteria for major bleeding was classified as a minor bleeding complication.
At each follow-up visit (3 and 12 months), patients underwent assessment of their right heart function (echocardiography), functional status (6-minute walk test), and quality of life (questionnaires). The clinical personnel who performed assessment of these outcomes were unaware of the treatment assignments.
STATISTICAL ANALYSIS.
The sample size was calculated on the basis of the primary hypothesis to determine USAT as superior to standard catheterization in the context of thrombus load reduction measured by percentage change in CT obstruction index. The sample-size assumptions were modeled after a methodically similar trial, which estimated SCDT and USAT thrombus clearance to be 43% and 64.5%, respectively (23). Per in vitro experiments, USAT has been shown to increase the degree of thrombolysis by >50% (6,7). The sample size to detect an improvement of at least 50% in the USAT group in comparison with the SCDT group with 80% power and a significance level of 0.05 was estimated at 36 patients per treatment group. We accounted for an attrition size of about 10% to 15% in the case of missing #48-hour repeated CTA, for a total of 82 patients.
Data management and monitoring have been previously described (15). All statistical analysis was done in an intention-to-treat fashion. For all statistical tests, a P value < 0.05 was considered to indicate statistical significance.
Continuous data are presented as mean ± SD or, in case of skewed distribution, as median values with ranges. Comparison of binary data between the groups was performed using the Fisher exact test. Within-group ordinal data were compared using the 2-sided Wilcoxon signed rank test. Between-group continuous data were compared using the Wilcoxon rank sum test.
Multivariable linear regression was used to test for significant predictors of PA thrombus score reduction, in which case clinically relevant predictors were chosen a priori (lysis dose and lysis time). A post hoc analysis of the subgroups of patients who received #8 hours of lysis was deemed necessary to investigate the effect of shorter lysis time on thrombus and RV/LV ratio reduction, on the basis of the results of the OPTALYSE PE trial (10), published while SUNSET sPE was enrolling, indicating favorable outcomes for USAT in 6-hour lysis protocols. Interobserver agreement for the computed tomography–evaluated thrombus scores and RV/LV ratios between the 2 investigators (J.L. and J.S.) was assessed using intraclass correlation coefficient (ICC) estimates and their 95% confidence intervals on the basis of mean rating, consistency, and a 2-way mixed-effects model. All statistical analyses were performed using Stata version 15 (StataCorp). Similarly, significance was defined as P < 0.05.
RESULTS
PATIENT CHARACTERISTICS.
From June 2016 through January 2020, a total of 82 patients were enrolled at 3 sites. Of these patients, 41 were randomly assigned to USAT and 41 to SCDT. One patient (USAT), despite providing informed consent, refused to undergo a 48-hour CT scan, so 81 patients were included in the analysis (Figure 1). Demographics, comorbidities, risk factors, and PE severity were similar between the groups (Table 1).
FIGURE 1. Flowchart.
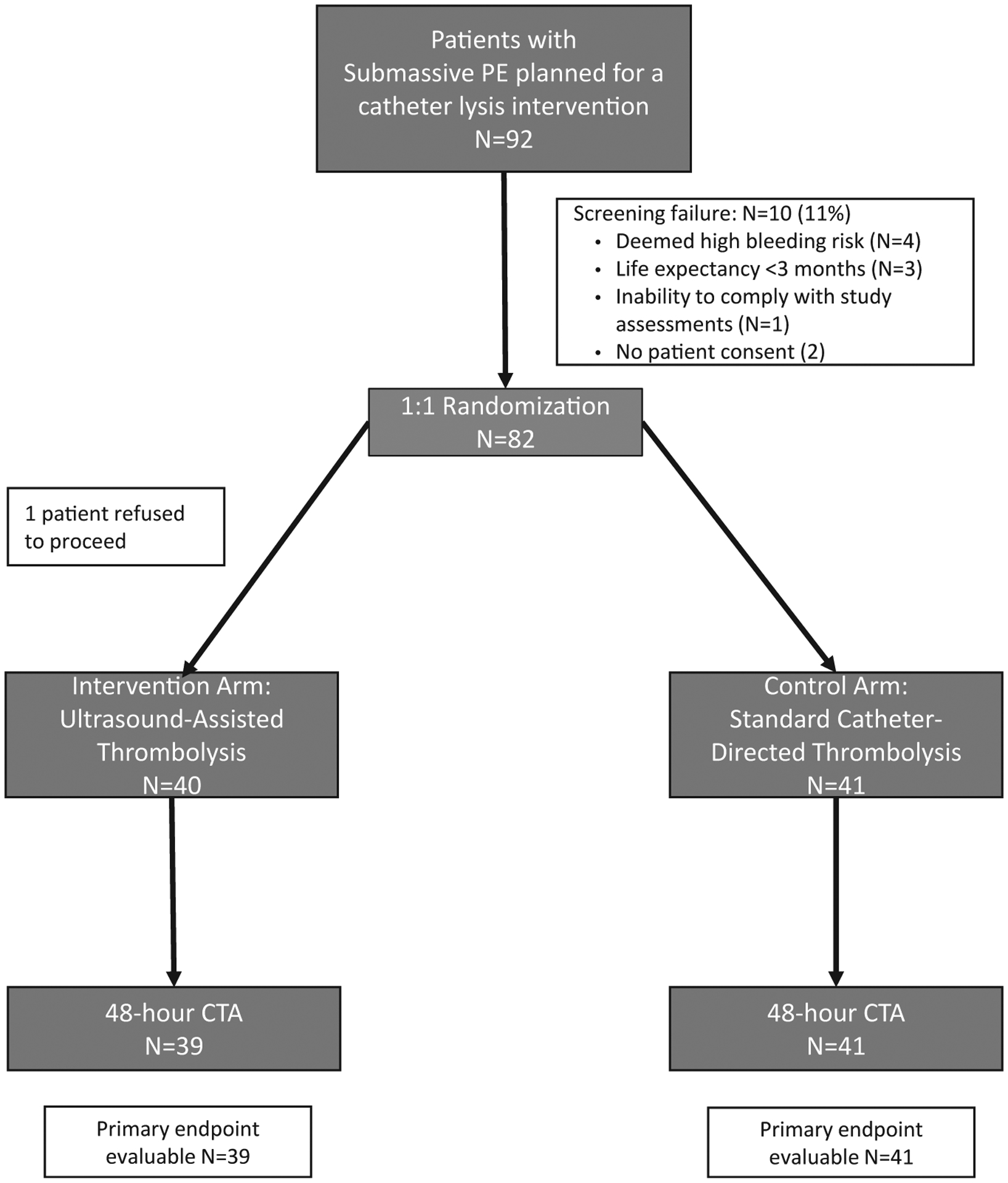
CTA = computed tomographic angiography; PE = pulmonary embolism.
TABLE 1.
Characteristics of the Patients at Baseline
| USAT (n = 40) | SCDT (n = 41) | Total (n = 81) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, yrs | 0.28 | |||
| Mean ± SD | 52 ± 13 | 55 ± 14 | 53 ± 14 | |
| Median (IQR) | 55 (21) | 54 (19) | 54 (18) | |
| Male | 23 (58) | 20 (49) | 43 (53) | 0.51 |
| White race | 25 (63) | 29 (71) | 54 (67) | 0.49 |
| Body mass index, kg/m2 | 0.59 | |||
| Mean ± SD | 37 ± 8 | 37 ± 9 | 40 ± 12 | |
| Median (IQR) | 35 (11) | 36 (10) | 35 (11) | |
| Comorbidities and risk factors | ||||
| Systemic hypertension | 15 (38) | 19 (46) | 34 (42) | 0.50 |
| Active smoking | 7 (18) | 11 (27) | 18 (22) | 0.42 |
| Pulmonary disease | 3 (8) | 3 (7) | 6 (7) | 0.65 |
| On home oxygen | 2 (5) | 0 (0) | 2 (3) | 0.24 |
| Coronary artery disease | 4 (10) | 5 (12) | 9 (11) | 0.52 |
| Cardiac heart failure | 1 (3) | 1 (2) | 2 (3) | 0.75 |
| GFR on admission, ml/min/1.73 m2 | 0.20 | |||
| Mean ± SD | 82 ± 26 | 75 ± 26 | 78 ± 28 | |
| Median (IQR) | 81 (31) | 68 (27) | 75 (29) | |
| Known hypercoagulable condition | 2 (5) | 0 | 2 (3) | 0.24 |
| Malignancy | 2 (5) | 2 (5) | 4 (5) | 0.68 |
| Prior DVT | 3 (8) | 9 (22) | 12 (15) | 0.06 |
| Prior PE | 5 (13) | 4 (10) | 9 (11) | 0.48 |
| Oral contraceptive use | 4 (10) | 3 (7) | 7 (9) | 0.49 |
| Recent travel (within 30 d) | 5 (13) | 5 (12) | 10 (12) | 0.615 |
| Acute DVT on presentation | 24 (62) | 27 (66) | 51 (64) | 0.43 |
| PE severity | ||||
| High intermediate risk | 38 (95) | 37 (90) | 75 (93) | 0.35 |
| Syncope at or before admission | 7 (18) | 8 (20) | 15 (19) | 0.52 |
| Troponin, μg/L* | 0.18 | |||
| Mean ± SD | 0.34 ± 0.39 | 0.73 ± 1.75 | 0.54 ± 1.28 | |
| Median (IQR) | 0.20 (0.42) | 0.28 (0.58) | 0.25 (0.46) | |
| Brain natriuretic peptide, ng/L | 0.64 | |||
| Mean ± SD | 629 ± 1,232 | 517 ± 817 | 570 ± 1,029 | |
| Median (IQR) | 227 (246) | 266 (497) | 255 (357) | |
| Baseline RV/LV ratio by CT | 0.08 | |||
| Mean ± SD | 1.54 ± .30 | 1.69 ± .44 | 1.62 ± .39 | |
| Median (IQR) | 1.53 (0.42) | 1.68 (0.55) | 1.61 (0.5) | |
| Baseline pulmonary obstruction score | 0.14 | |||
| Mean ± SD | 31 ± 4 | 33 ± 4 | 32 ± 4 | |
| Median (IQR) | 32 (4) | 33 (6) | 32 (5) | |
| Baseline pulmonary obstruction index | 0.29 | |||
| Mean ± SD, % | 71 ± 8 | 73 ± 7 | 72 ± 8 | |
| Median (IQR) | 72 (10) | 72 (10) | 72 (10) |
Values are mean ± SD, median (IQR), or n (%).
Highest value within 24 hours of admission.
CT = computed tomography; DVT = deep vein thrombosis; GFR = glomerular filtration rate; IQR = interquartile range; LV = left ventricular; PE = pulmonary embolism; RV = right ventricular; SCDT = standard catheter-directed thrombolysis; USAT = ultrasound-assisted thrombolysis.
TREATMENT DETAILS.
Procedures were performed by a total of 19 interventionalists. The mean total dose of alteplase for USAT was 19 ± 7 mg and for SCDT was 18 ± 7 mg (P = 0.53) over 14 ± 6 hours and 14 ± 5 hours, respectively (P = 0.99) (Table 2). Three patients within the USAT group had interruption of lytic therapy resulting from EKOS alarming, indicating catheter or wire kink or malpositioning. Two of these patients continued lysis without ultrasound (EKOS was turned off); EKOS therapy was resumed in the third. Additionally, 2 patients within the USAT group had early termination of therapy as a result of catheter dislodgement. These 2 patients had lysis times of 4 and 6 hours.
TABLE 2.
Treatment and Procedural Details
| USAT (n = 40) | SCDT (n = 41) | Total (n = 81) | P Value | |
|---|---|---|---|---|
| Contraindications to lysis | 0.37 | |||
| Minor | 8 (20) | 6 (15) | 14 (17) | |
| Major | 0 | 0 | ||
| PA systolic pressure, mm Hg | 55 ± 15 | 57 ± 16 | 56 ± 15 | 0.55 |
| PA diastolic pressure, mm Hg | 26 ± 10 | 25 ± 8 | 25 ± 9 | 0.63 |
| PA mean pressure, mm Hg | 37 ± 10 | 37 ± 10 | 37 ± 10 | 0.91 |
| On-table bolus tPA | 23 (58) | 26 (63) | 49 (61) | 0.38 |
| On-table bolus tPA dose, mg* | 0.53 | |||
| Mean | 3 ± 3 | 3 ± 4 | 3 ± 4 | |
| Median (IQR) | 2 (4) | 2 (4) | 2 (4) | |
| Total tPA dose | 0.53 | |||
| Mean | 19 ± 7 | 18 ± 7 | 19 ± 7 | |
| Median (IQR) | 20 (11) | 18 (11) | 19 (10) | |
| Total hours of tPA drip | 0.99 | |||
| Mean | 14 ± 5 | 14 ± 6 | 14 ± 6 | |
| Median (IQR) | 12 (9) | 14 (10) | 13 (11) |
Values are n (%), mean ± SD, median (IQR).
For those who received on-table tPA.
IQR = interquartile range; PA = pulmonary artery; tPA = tissue plasminogen activator; other abbreviations as in Table 1.
INTEROBSERVER AGREEMENT FOR PA THROMBUS SCORE AND RV/LV RATIO.
Seventy-one CT angiographic scans (89%) were used to measure interrater reliability using ICCs. The mean thrombus load reduction for reviewer 1 was 20% ± 14% and 21% ± 14% for reviewer 2. The mean differences in baseline RV/LV ratio and posttreatment RV/LV ratio for reviewer 1 were 0.44 ± 0.39 and 0.47 ± 0.34 for reviewer 2. There was excellent agreement between the 2 reviewers for thrombus score reduction (ICC = 0.93) and good agreement between the 2 reviewers for measuring difference in RV/LV ratio (ICC = 0.84).
PRIMARY ENDPOINT ANALYSIS.
Eighty patients (USAT, n = 39; SCDT, n = 41) had pre- and postintervention CT scans to evaluate the primary endpoint. In the USAT group, the mean raw PA thrombus score was reduced from 31 ± 4 at baseline to 22 ± 7 (P < 0.001). In the SCDT group, the score was reduced from 33 ± 4 to 23 ± 7 (P < 0.001). There was no statistically significant difference in mean PA raw thrombus score reduction between the 2 groups (9 ± 6 vs 10 ± 6, respectively; P = 0.76) (Table 3).
TABLE 3.
Trial Outcomes
| USAT (n = 40) | SCDT (n = 41) | P Value | |
|---|---|---|---|
| Primary outcome | |||
| Pulmonary obstruction index reduction, % | 21 ± 13 | 22 ± 13 | 0.77 |
| Pulmonary obstruction score reduction | 9 ± 6 | 10 ± 6 | 0.76 |
| Secondary outcomes | |||
| RV/LV ratio reduction* | 0.37 ± 0.34 | 0.59 ± 0.42 | 0.01 |
| ICU stay | |||
| Mean | 4.1 ± 8.8 | 2.4 ± 1.2 | 0.23 |
| Median (IQR) | 2 (2–4) | 2 (2–3) | 2 (2–3) |
| Hospital stay | |||
| Mean | 7.7 ± 8.7 | 4.6 ± 1.8 | 0.03 |
| Median (IQR) | 6 (4–8) | 4 (3–6) | 6 (4–7) |
| Decompensation | 0 | 0 | Total 0 |
| Major bleeding events | 2 | 0 | Total 2 (2.5) |
| Minor bleeding events | 3 | 0 | Total 3 (3.7) |
| In-hospital death | 1 | 0 | Total 1 (1.2) |
Values are mean ± SD, median (IQR), or n (%).
Five USAT and 3 SCDT patients had no RV/LV ratio improvement; they were included in the analysis.
ICU = intensive care unit; other abbreviations as in Table 1.
The baseline CT obstruction index in the USAT group was 71% ± 8% and dropped after CDT to 50% ± 17% (P < 0.001). In the SCDT group, baseline CT obstruction score was 73% ± 7% and dropped after CDT to 51% ± 15% (P < 0.001). The mean thrombus load reduction was 21% 13% for USAT and 22% ± 13% for SCDT (P = 0.77) (Central Illustration). The results remained similar even after excluding the 5 USAT cases in which an alarm or early discontinuation occurred.
CENTRAL ILLUSTRATION. Primary Endpoint of the Standard Versus Ultrasound-Assisted Catheter Thrombolysis for Submassive Pulmonary Embolism Trial.
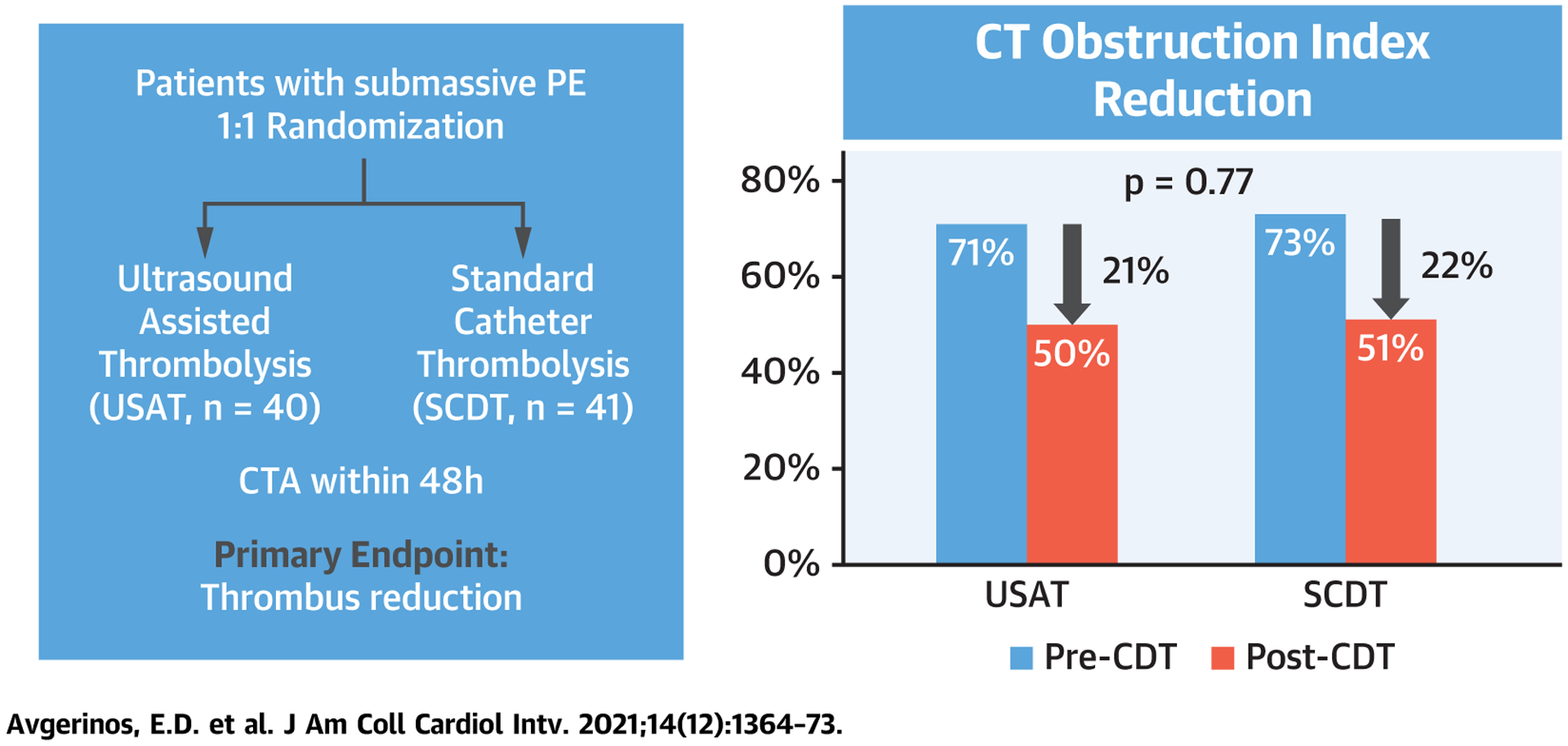
CT = computed tomographic; CTA = computed tomographic angiography; SCDT = standard catheter-directed thrombolysis; USAT = ultrasound-assisted thrombolysis.
Overall, lysis tPA dose (P = 0.561) and lysis time (P = 0.824) were not predictors of increased thrombus load reduction. Looking at each group separately, there was no correlation between lysis time (P = 0.11 for USAT, P = 0.10 for SCDT) or total alteplase dose (P = 0.39 for USAT, P = 0.88 for SCDT) and PA thrombus score reduction. Post hoc, we subanalyzed 20 patients (8 USAT and 12 SCDT) who received ≤8 hours of lysis, and apparently thrombus reduction was higher in the USAT group (28% ± 19% vs 14% ± 7%; P = 0.03).
SECONDARY ENDPOINTS.
In the USAT group (n = 39), the mean RV/LV ratio was reduced from 1.5 ± 0.3 at baseline to 1.2 ± 0.2 (P < 0.001) and in the SCDT group (n = 41) from 1.7 ± 0.4 to 1.1 ± 0.2 (P < 0.001). In 5 USAT and 3 SCDT patients, there was no RV/LV ratio improvement. The mean difference in RV/LV ratio from baseline to 48 hours was 0.37 ± 0.34 in USAT group and 0.59 ± 0.42 in the SCDT group (P = 0.01). The result remained similar and significant even after excluding the 5 USAT cases in which an alarm or early discontinuation occurred and even when analyzing patients who received #8 hours of lysis. The average ICU stay for the entire cohort was 2 days (range 2 days-3 days), similar between groups; however, hospital stay was shorter for the SCDT group at 4.6 ± 1.8 days versus 7.7 ± 8.7 days in the USAT group (P = 0.03) (Table 3).
SAFETY OUTCOMES.
Major bleeding occurred in 2 patients, both in the USAT group. One was a 71-year-old woman who developed a hemorrhagic stroke manifesting as hemianopia with a large right occipital lobe hemorrhage. This was managed conservatively and at most recent follow-up was slowly diminishing. The other major bleeding event was epistaxis and vaginal bleeding in a 53-year-old patient with baseline menometrorrhagia, who eventually required transfusion of 2 U blood. Both patients had received higher tPA doses (~28 mg).
Minor bleeding occurred in 3 patients, all in the USAT group. Two patients had hematemesis postprocedurally, and 1 patient had thigh and flank hematoma. These were all managed conservatively without need for blood transfusion.
One patient was diagnosed with heparin-induced thrombocytopenia and was converted to argatroban and eventually warfarin.
There was 1 in-hospital death on day 58 of a patient enrolled in the USAT group. The patient had hypersensitivity pneumonitis and was diagnosed with bilateral PE, which was thought to be the source of her respiratory deterioration. Her pneumonitis persisted and she never clinically improved.
DISCUSSION
In the SUNSET sPE trial, patients with sPE undergoing USAT did not have improved pulmonary arterial thrombus reduction in comparison with standard (nonultrasound) multiple-side-hole catheter thrombolysis. Although both technical alternatives produced significant RV function improvement, the SCDT group demonstrated a superior RV/LV ratio reduction. Both techniques exhibited good safety profiles.
CDT has been introduced in the management of non-low-risk PE to mitigate the high rate of bleeding events associated to systemic thrombolysis, while maintaining its potential effectiveness, against anticoagulation alone, in quick RV dysfunction reversal (1). Our trial confirmed this notion, demonstrating an average RV/LV ratio reduction of 30% within 48 hours and an overall major bleeding rate of 2.5%; this is one of the lowest reported bleeding rates among several interventional trials with or without thrombolytics (aspiration thrombectomy) (5,8–10,24). In a similar population of patients with sPE enrolled in PEITHO (PEITHO Pulmonary Embolism Thrombolysis Study), systemic thrombolysis was associated with a major bleeding rate of 11.5% (25).
USAT emerged approximately a decade ago in the PE field as an enhanced thrombolytic technique, potentially superior to standard catheter tPA infusion (12). The ULTIMA (Ultrasound Accelerated Thrombolysis of Pulmonary Embolism) randomized trial established USAT as superior to heparin alone in early reversal of RV dysfunction, leading to Food and Drug Administration approval for the EKOS catheter as the only approved thrombolytic catheter for PE, and boosted its use globally, despite its small sample size (5,8,11). More recent research has established its efficacy and safety predominately in sPE but also in massive PE (5), and a randomized trial against anticoagulation looking at clinical endpoints is under way (26). However, there has been ongoing controversy regarding whether the addition of ultrasound truly adds an enhanced lytic effect and whether this translates to a clinical benefit that justifies the 10-fold higher cost of the catheter and the capital investment (11,17). A similar controversy in the deep vein thrombosis intervention was resolved by the BERNUTIFUL (Ultrasound-Enhanced Thrombolysis Versus Standard Catheter Directed Thrombolysis for Ilio-femoral Deep Vein Thrombosis) randomized trial, also with a small sample size, which showed no added benefit in thrombus removal when ultrasound was used (23). In the PE field, the evidence supporting the use of USAT over traditional SCDT has been limited by multiple small, equivocal retrospective studies and lack of prospective comparative outcomes data (5,12–15). The first study to imply equivalence was the prospective PERFECT (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) registry, which did not show any hemodynamic or clinical difference between USAT and SCDT (13). More recently, another multicenter retrospective study did not confirm any difference in short-term hemodynamic and longer term functional outcomes (27). Multiple series have yielded similar conclusions, but with small numbers of clinical endpoints they have been statistically underpowered to prove superiority. In a recent meta-analysis compiling 1,031 patients, “clinical success” as defined by the investigators was higher in those who underwent USAT for massive PE (83.1% vs 70.8%), with no difference in patients with sPE (5).
Acknowledging that using hard clinical endpoints (eg, mortality) in the design of a randomized trial would require a sample of several hundreds of patients, the SUNSET sPE trial was specifically powered to show that ultrasound can increase thrombus clearance in human PAs. The trial confirmed similar thrombus reduction, roughly 21% within 48 hours, after an average 14-hour alteplase infusion time and a total treatment dose of 19 mg alteplase. The OPTALYSE PE trial (10) demonstrated efficacy of the EKOS system with shorter lysis time, and it could be argued that SCDT would not perform as well within such a short time frame. After the 34th patient was enrolled, despite our recommendation to operators to adhere to a lower dose and time protocol, the change in practice was near obsolete. Our subgroup analysis for patients who received shorter (≤8 hours) lysis infusion did show an improved thrombus reduction with USAT, but this was likely an erroneous underpowered result, and it otherwise did not correspond to a similar RV/LV ratio response; but further studies may be needed. In the analysis of the entire cohort, thrombus reduction did not seem to correlate to the alteplase dose or lysis time, at least in the context of a “terminate upon vital sign improvement” real-world practice, likely indicating inherent differences in thrombus chronicity. This is in contrast to the OPTALYSE PE trial, in which higher tPA dose resulted in higher thrombus reduction. As our procedural termination was clinically driven and not protocolized on dose or time, as in OPTALYSE PE, the results may not be comparable. Shorter times are probably adequate for both treatment groups. Additionally, RV/LV ratio reduction seemed to be superior in the SCDT cohort, irrespective of lysis dose and duration. Although there is no apparent explanation for this observation, the study was not designed for this outcome, so it should be viewed with caution. The same applies for the longer hospital stay in the USAT group.
STUDY LIMITATIONS.
The study was powered to detect an improvement of at least 50% in the USAT group; thus, detection of smaller differences, if present, would require a larger sample size. Interventionalists were allowed a liberal technical protocol, leading to tPA dose and time variability. Although a fixed dose and dripping time would have eliminated tPA dosing bias, this would not be a pragmatic trial design. An endpoint of thrombolysis termination using physician judgement of hemodynamic or clinical improvement is real-world practice; if USAT were more potent, our results would have demonstrated a need for lower tPA dose or shorter infusion period, but this was not seen. Another limitation is the long enrollment period of approximately 4 years, which may have introduced biases related to change in the overall care of patients with PE. During the enrollment period, multiple competing trials started interfering, and we noticed an overall declining volume as our interventional management became protocolized and standardized, adhering to the PERT Consortium recommendations. Finally, our results should not be generalized to the massive PE population.
CONCLUSIONS
This is the first randomized trial introducing head-to-head comparison of interventional techniques in an attempt to guide a cost-conscious treatment algorithm in the management of PE patients. USAT may not confer additional benefits to standard catheter thrombolytic techniques.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
CDT has been increasingly used in both sPE and massive PE as a decompensation prevention and potentially lifesaving procedure. It is unproved whether USAT using the EKOS catheter is superior to standard (non-ultrasound-accelerated) CDT using traditional multiple-side-hole catheters in the treatment of PE.
WHAT IS NEW?
Patients with sPE undergoing USAT had similar pulmonary arterial thrombus reduction compared with those undergoing SCDT, using comparable mean lytic dose and duration of lysis.
WHAT IS NEXT?
USAT for sPE may not confer additional benefits to standard catheter thrombolytic techniques, but larger trials using shorter lysis times (4–6 hours) may be needed.
ACKNOWLEDGMENTS
The SUNSET sPE collaborators were: M. Gladwin, P. Lamberty, C. Kabrhel, A.J. Klein, M.S. Makaroun, C.E. Miller, A. Mohapatra, I. Ocak,H. Phelos, and R. Sachdeva. The following members also participated in the SUNSET sPE trial: Georges Al-Khoury and Michael Madigan (University of Pittsburgh Medical Center); Nathan Liang (University of Pittsburgh Medical Center); biostatistics: Larry Fish and Heather Phelos (University of Pittsburgh Medical Center); research coordinators: Julianna Sheline and Judith Brimmeier (University of Pittsburgh Medical Center).
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr. Avgerinos is a member of the Speakers Bureau for Boston Scientific; and is a consultant for AngioDynamics and BD Medical. Dr. Chaer is a member of the Speakers Bureau for Boston Scientific. Dr. Jaber is a consultant for Inari. Dr. Ross is a member of the Peripheral Intervention Vascular Senior Medical Council for Boston Scientific Corporation. Dr. Rivera-Lebron is a consultant for Bristol Myers Squibb. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- CDT
catheter-directed thrombolysis
- CT
computed tomographic
- CTA
computed tomographic angiography
- ICC
intraclass correlation coefficient
- ICU
intensive care unit
- LV
left ventricular
- PA
pulmonary artery
- PE
pulmonary embolism
- RV
right ventricular
- SCDT
standard catheter-directed thrombolysis
- sPE
submassive pulmonary embolism
- tPA
tissue plasminogen activator
- USAT
ultrasound assisted thrombolysis
Footnotes
APPENDIX For a description of scoring, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Giri J, Sista AK, Weinberg I, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation 2019;140(20): e774–801. [DOI] [PubMed] [Google Scholar]
- 2.Gayou EL, Makary MS, Hughes DR, et al. Nationwide trends in use of catheter-directed therapy for treatment of pulmonary embolism in Medicare beneficiaries from 2004 to 2016. J Vasc Interv Radiol 2019;30(6):801–6. [DOI] [PubMed] [Google Scholar]
- 3.Stein PD, Matta F, Hughes MJ. Thrombolysis in submassive pulmonary embolism and acute cor pulmonale. Am J Cardiol 2020;131:109–14. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311(23):2414–21. [DOI] [PubMed] [Google Scholar]
- 5.Avgerinos ED, Saadeddin Z, Abou Ali AN, et al. A meta-analysis of outcomes of catheter-directed thrombolysis for high- and intermediate-risk pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2018;6(4):530–40. [DOI] [PubMed] [Google Scholar]
- 6.Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasoundpotentiated fibrinolysis in vitro. Blood 1993; 81(10):2636–43. [PubMed] [Google Scholar]
- 7.Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol 1995;21(3):419–24. [DOI] [PubMed] [Google Scholar]
- 8.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129(4):479–86. [DOI] [PubMed] [Google Scholar]
- 9.Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, lowdose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. J Am Coll Cardiol Intv 2015;8(10):1382–92. [DOI] [PubMed] [Google Scholar]
- 10.Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. J Am Coll Cardiol Intv 2018; 11(14):1401–10. [DOI] [PubMed] [Google Scholar]
- 11.Beyer SE, Shanafelt C, Pinto DS, et al. Utilization and outcomes of thrombolytic therapy for acute pulmonary embolism. Chest 2020;157(3): 645–53. [DOI] [PubMed] [Google Scholar]
- 12.Lin PH, Annambhotla S, Bechara CF, et al. Comparison of percutaneous ultrasound-accelerated thrombolysis versus catheter-directed thrombolysis in patients with acute massive pulmonary embolism. Vascular 2009;17 suppl 3:S137–47. [DOI] [PubMed] [Google Scholar]
- 13.Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 2015;148(3):667–73. [DOI] [PubMed] [Google Scholar]
- 14.Avgerinos ED, Abou Ali AN, Liang NL, et al. Predictors of failure and complications of catheter-directed interventions for pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2017;5(3):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao G, Xu H, Jason W, et al. Ultrasound-assisted versus conventional catheter directed thrombolysis for acute pulmonary embolism: a multi-center comparison of patient-centered outcomes. Vasc Med 2019;24(3):234–40. [DOI] [PubMed] [Google Scholar]
- 16.Bloomer T, El Hayek G, McDaniel MC, et al. Safety of catheter directed thrombolysis for massive and submassive pulmonary embolism: results of a multicenter registry and meta-analysis. Catheter Cardiovasc Interv 2017;89(4): 754–60. [DOI] [PubMed] [Google Scholar]
- 17.Avgerinos ED, Chaer RA, for the SUNSET sPE Collaborators. Standard vs ultrasound-assisted thrombolysis for acute pulmonary embolism: awaiting the verdict. Vasc Med 2019;24(6):547–8. [DOI] [PubMed] [Google Scholar]
- 18.Wissam JA, McDaniel MC. Catheter-based embolectomy for acute pulmonary embolism: devices, technical considerations, risks, and benefits. Interv Cardiol Clin 2018;7(1):91–101. [DOI] [PubMed] [Google Scholar]
- 19.Avgerinos ED, Mohapatra A, Rivera-Lebron B, et al. Design and rationale of a randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2018; 6(1):126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera-Lebron B, McDaniel M, Ahrar K, et al. , for the PERT Consortium. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT Consortium. Clin Appl Thromb Hemost 2019;25: 1076029619853037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qanadli SD, El Hajjam M, Vieillard-Baron A, et al. New CT index to quantify arterial obstruction in pulmonary embolism. AJR Am J Roentgenol 2001;176(6):1415–20. [DOI] [PubMed] [Google Scholar]
- 22.Mastora I, Remy-Jardin M, Masson P, et al. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 2003;13(1):29–35. [DOI] [PubMed] [Google Scholar]
- 23.Engelberger RP, Spirk D, Willenberg T, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv 2015;8(1): e002027. [DOI] [PubMed] [Google Scholar]
- 24.Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. J Am Coll Cardiol Intv 2019;12(9):859–69. [DOI] [PubMed] [Google Scholar]
- 25.Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370(15): 1402–11. [DOI] [PubMed] [Google Scholar]
- 26.The Higher-Risk Pulmonary Embolism Thrombolysis Study (HI-PEITHO). Paper presented at: 6th Annual PERT Consortium Scientific Symposium; October 23–24, 2020. [Google Scholar]
- 27.Allen S, Chan L, Masic D, et al. Comparison of outcomes in catheter-directed versus ultrasound assisted thrombolysis for management of submassive pulmonary embolism. Thromb Research 2021;202:96–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


