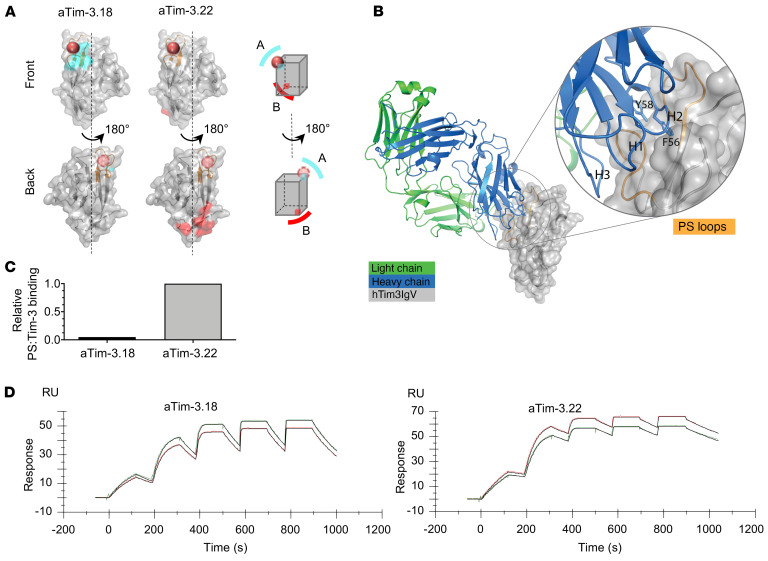
Figure 3. Two anti–Tim-3 antibodies bind with high affinity to Tim-3 but target 2 nonoverlapping epitopes.
(A) Yeast display epitope mapping of aTim-3 mAbs to human Tim-3 (hTim-3): aTim-3.18 mAb binds to hTim-3 (A, shown in cyan) on the PS binding site (orange sphere), while aTim-3.22 binds the opposite face of hTim-3 (B, red) away from the PS binding site. (B) X-ray crystal structure of aTim-3.18:hTim-3 complex reveals heavy chain CDR2 (blue) binds the PS binding loops (orange), with residues F56 and Y58 inserted into the PS binding pocket. CDR2, complementarity-determining region 2; h, human; IgV, Ig variable. (C) Cell-free PS blocking assay showing relative binding of PS liposomes to hTim-3 saturated with aTim-3.18 or aTim-3.22. (D) Kinetic affinity measurements of aTim-3.18 and aTim-3.22 mAbs. Surface plasmon resonance sensorgrams of hTim-3 binding in increasing concentrations to aTim-3.18 (left) or aTim-3.22 (right) mAbs (data in color, fit in black). Each mAb was captured in duplicate on different flow cells.