Nucleophilic addition with Grignard reagents on cyclic N,O-acetalsa.
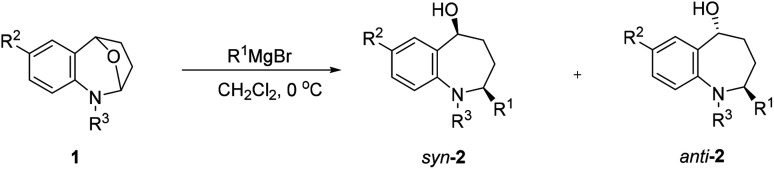
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | N,O-Acetals | R1 | R2 | R3 | Time | 2 | Yield (%) | syn/antib |
| 1 | 1a | Me | H | Allyl | 10 min | 2a | 96 | 69/31 |
| 2 | 1a | Et | H | Allyl | 10 min | 2b | 97 | 91/9 |
| 3 | 1a | i-Pr | H | Allyl | 7 h | 2c | 88 | 100/0 |
| 4 | 1a | Cy | H | Allyl | 18 h | 2d | 83 | 100/0 |
| 5 | 1a | Allyl | H | Allyl | 10 min | 2e | 98 | 0/100 |
| 6 | 1a | Ph | H | Allyl | 10 min | 2f | 98 | 100/0 |
| 7 | 1b | Me | Me | Allyl | 10 min | 2g | 91 | 88/12 |
| 8 | 1b | Allyl | Me | Allyl | 10 min | 2h | 90 | 0/100 |
| 9 | 1c | Me | Cl | Allyl | 10 min | 2i | 92 | 67/33 |
| 10 | 1c | Allyl | Cl | Allyl | 10 min | 2j | 91 | 0/100 |
| 11 | 1d | Me | H | Me | 10 min | 2k | 92 | 68/32 |
| 12 | 1d | i-Pr | H | Me | 5 min | 2l | 91 | 100/0 |
| 13 | 1e | Me | H | H | 1 h | 2m | 80 | 91/9 |
| 14 | 1e | Allyl | H | H | 10 min | 2n | 81 | 75/25 |
Unless indicated otherwise, the reaction was carried out on 1.0 mmol scale in DCM (10 mL).
Diastereoisomeric ratios were determined by 1H NMR analysis of the mixture, see the ESI for details.
