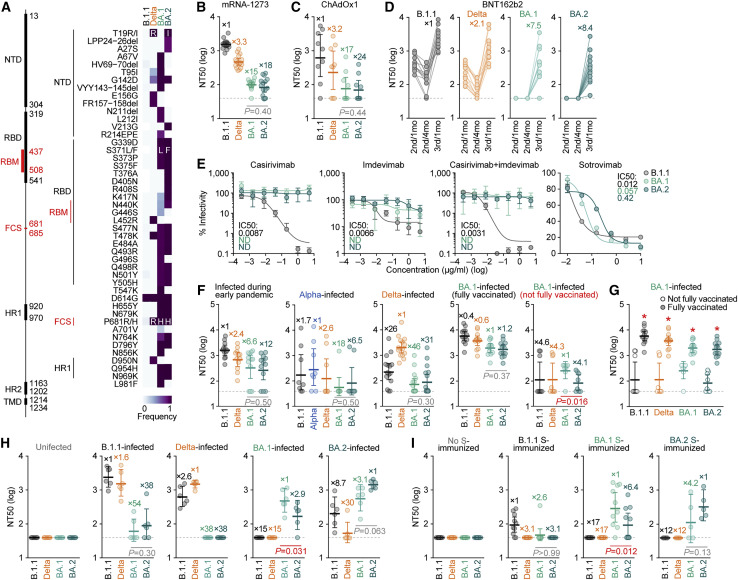
Figure 2.
Immune resistance of BA.2
(A) Amino acid substitutions in S. Left, primary structure and domains of the virus. The numbers indicate the amino acid positions. NTD, N-terminal domain; RBM, receptor-binding motif; HR, heptad repeat; TMD, transmembrane domain. Right, heatmap showing the frequency of amino acid substitutions. Substitutions detected in >10% of sequences of any lineage are shown.
(B–I) Neutralization assays. Neutralization assays were performed with pseudoviruses harboring the S proteins of B.1.1 (the D614G-bearing ancestral virus), Delta, BA.1 and BA.2, and the following sera and monoclonal antibodies.
(B) mRNA-1273 vaccine (16 donors).
(C) ChAdOx1 vaccine (9 donors).
(D) BNT162b2 vaccine (13 donors). 2nd/1mo, 1 month after the 2nd dose; 2nd/4mo, 4 months after the 2nd dose; 3rd/1mo, 1 month after the 3rd dose.
(E) Therapeutic monoclonal antibodies (casirivimab, imdevimab, casirivimab + imdevimab, and sotrovimab). IC50, 50% inhibitory concentration; ND, not determined.
(F and G) Convalescent sera from individuals infected with an early pandemic virus (until May 2020) (12 donors), Alpha (8 donors), Delta (15 donors), or BA.1 [13 fully vaccinated donors or 8 not fully vaccinated donors].
(H) Sera from uninfected, B.1.1-infected, Delta-infected, BA.1-infected, and BA.2-infected hamsters at 16 d.p.i. (6 hamsters per each group).
(I) Sera from mice immunized with empty vector-transfected cells (10 mice), cells expressing B.1.1 S (10 mice) or BA.1 S (H, right; 10 mice) were used.
In (B)–(D) and (F)–(I), assays with each serum sample were performed in triplicate to determine the 50% neutralization titer (NT50). Each dot represents one NT50 value, and the geometric mean and 95% CI are shown. The numbers indicate the fold changes of resistance versus each antigenic variant. The horizontal dashed line indicates the detection limit (40-fold). Statistically significant differences between BA.1 and BA.2 were determined by two-sided Wilcoxon signed-rank tests (B, C, F, H, and I) or two-sided Mann-Whitney U tests (G, ∗ p < 0.05). Information on the vaccinated/convalescent donors is summarized in Table S2.
In (E), the assays for each concentration were performed in triplicate, and the presented data are expressed as the average ± SD.
See also Table S2.