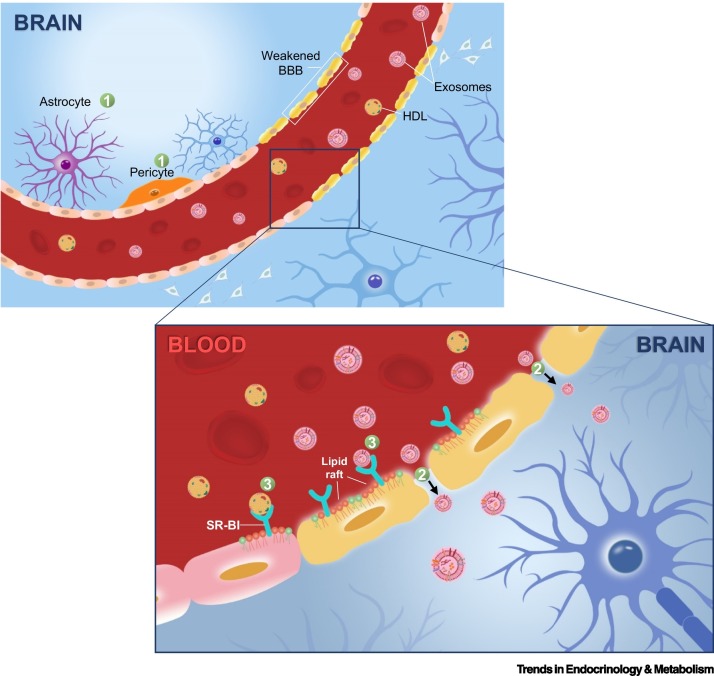
Figure 3.
Hematogenous route of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neurotropism.
The hematogenous route of viral neurotropism comprises transcellular, paracellular, and Trojan horse modes of entry [116]. Transcellular mode (1) of entry requires viral binding and cellular uptake by specific cell types constituting the natural barriers of the brain, which requires these cells to possess plasma membrane receptors (e.g., ACE2) capable of recognizing and binding to the spike proteins of SARS-CoV-2. Some of these cell types enabling SARS-CoV-2 neurotropism transcellularly, as revealed by viral binding assays based on organoids and sectioned brain slices, include ChP epithelial cells of the BSCFB, as well as pericytes and astrocytes surrounding endothelial cells of the BBB [19., 20., 21.]. The paracellular mode (2) of entry entails a weakening of intercellular tight junctions between the cerebral vascular endothelial cells to facilitate an enhanced diffusion of substances across the BBB. The Trojan horse (3) mode of viral neurotropism typically involves leveraging host cells (e.g., blood cells) or endogenous lipid particles/vesicles, such as HDLs and exosomes that are central to this review, to achieve immune evasion from host’s surveillance in the process of traversing the BBB. The SR-BI receptor localized on lipid raft microdomains mediates the transmigration of HDLs and exosomes across the BBB [95]. Abbreviations: ACE2, angiotensin-converting enzyme 2; BBB, blood–brain barrier; BSCFB, blood–cerebrospinal fluid barrier; ChP, choroid plexus; HDL, high-density lipoprotein; SR-BI, scavenger receptor class B type I.