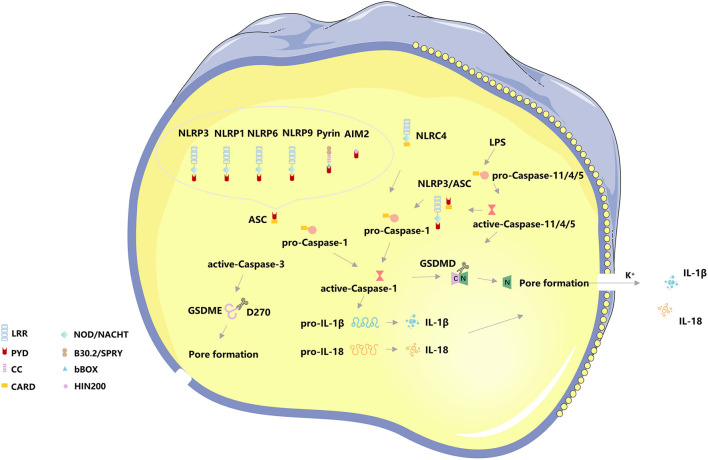
Figure 1.
The basic molecular mechanism of pyroptosis. The canonical pathway of pyroptosis, Nod-like receptors protein-3 (NLRP3), NLRP1, NLRP6, NLRP9, absent in melanoma 2 (AIM2), and Pyrin binds to the N-terminal PYD region of the apoptosis-associated speck-like protein (ASC) to activate ASC proteins through protein-protein interactions. The C-terminal CARD domain of ASC and the N-terminal CARD domain of pro-Caspase-1 combine to recruit active-Caspase-1. The binding complex of PRRs, ASC, and pro-Caspase 1 is termed the inflammasome. On the one hand, Caspase-1 recognizes pro-IL-1β and pro-IL-18, converts them into IL-1β and IL-18, and releases them extracellular to expand the inflammatory response, on the other hand, Caspase-1 shear Gasdermin family protein GSDMD to separate its N- and C- domains, N-terminal fragments are released to the membrane, mediating the formation of cell membrane pores, releasing inflammatory factors and inducing pyroptosis. NLRC4 can directly interact with pro-Caspase-1 via CARD-CARD to form active-Caspase-1 and induce pyroptosis. The non-canonical pathway of pyroptosis, Caspase4/5/11 can directly bind to lipopolysaccharide (LPS) in the cytoplasm and initiate pyroptosis following cleavage of GSDMD-induced membrane pore formation and subsequent cell membrane rupture. The K+ efflux caused by cell membrane pore formation induces activation of the NLRP3/ASC/ Caspase-1 pathway. In addition, Caspase-3 cleaves the Gasdermin family protein GSDME, releasing the N-terminal active fragment to the cell membrane, leading to pyroptosis.