Figure EV5. (related to Fig 5) Blocking the decoding of K27‐linked ubiquitylation signals impairs p97 substrate turnover.
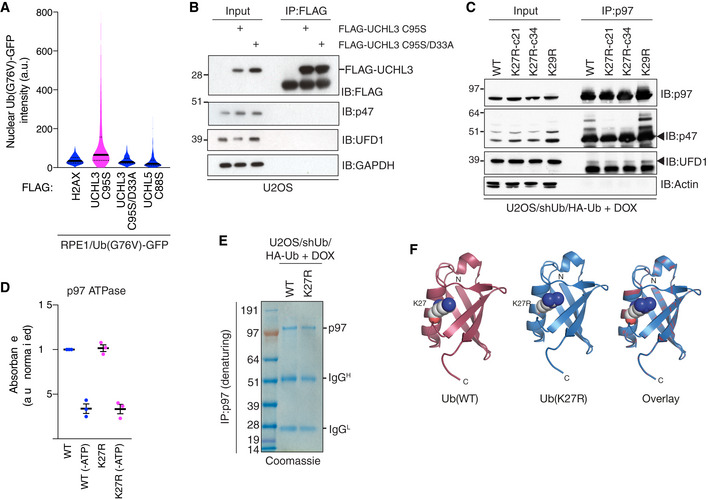
-
ARPE‐1 cells stably expressing Ub(G76V)‐GFP were transfected with indicated FLAG‐tagged expression constructs, fixed and immunostained with FLAG antibody. Levels of Ub(G76V)‐GFP in FLAG‐positive cells were quantified using QIBC (solid lines, median; dashed lines, quartiles; > 1,000 transfected cells analyzed per condition).
-
BU2OS cells transfected with FLAG‐UCHL3 constructs were subjected to FLAG IP followed by immunoblotting with indicated antibodies.
-
CDOX‐treated Ub replacement cell lines were subjected to p97 IP followed by immunoblotting with the indicated antibodies.
-
DATPase activity of p97 immobilized by IP under denaturing conditions from U2OS/shUb/HA‐Ub(WT) or U2OS/shUb/HA‐Ub(K27R) (mean ± SEM; n = 3 independent experiments).
-
ECoomassie staining of p97 IPs in (D).
-
FCrystal structure of human Ub (PDB ID: 5UJL; Castaneda et al, 2016) (left), modeled structure of a Ub(K27R) mutant (middle) using the PyMol mutagenesis tool, and their overlay (right). Amino acids at the Ub K27 position are highlighted by space‐filling representation.
Data information: Data are representative of three (A,D,E) and two (B,C) independent experiments with similar outcome.
