Abstract
Background and Aims
As the coronavirus disease 2019 (COVID‐19) pandemic spread worldwide in 2020, the number of patients requiring intensive care and invasive mechanical ventilation (IMV) has increased rapidly. During the pandemic, early recommendations suggested that tracheostomy should be postponed, as the potential benefits were not certain to exceed the risk of viral transmission to healthcare workers. The aim of this study was to assess the utility of tracheostomy in patients with COVID‐19‐related acute respiratory distress syndrome, in terms of patient and clinical characteristics, outcomes, and complications, by comparing between early and late tracheostomy.
Methods
A multicenter, retrospective observational study was conducted in Jönköping County, Sweden. Between 14 March 2020 and 13 March 2021, 117 patients were included. All patients ≥18 years of age with confirmed COVID‐19, who underwent tracheostomy were divided into two groups based on the timing of the procedure (≤/>7 days). Outcomes including the time on IMV, intensive care unit (ICU) length of stay, and mortality 30 days after ICU admission, as well as complications due to tracheostomy were compared between the groups.
Results
Early tracheostomy (<7 days, n = 56) was associated with a shorter median duration of mechanical ventilation (7 [12], p = 0.001) as well as a shorter median ICU stay (8 [14], p = 0.001). The most frequent complication of tracheostomy was minor bleeding. With the exception of a higher rate of obesity in the group receiving late tracheostomy, the patient characteristics were similar between the groups.
Conclusion
This study showed that early tracheostomy was safe and associated with a shorter time on IMV as well as a shorter ICU length of stay, implicating possible clinical benefits in critically ill COVID‐19 patients. However, it is necessary to verify these findings in a randomized controlled trial.
Keywords: acute respiratory distress syndrome, COVID‐19, ICU, tracheostomy
1. INTRODUCTION
In December 2019, an unknown form of pneumonia was reported in Wuhan, China. 1 Within the first weeks of 2020, the coronavirus disease 2019 (COVID‐19) had spread worldwide, causing a pandemic. 2 In May 2021, the World Health Organization reported over 152 million cases and three million deaths globally due to COVID‐19. 3 In Sweden, the number of cases peaked in April 2020 and then again in December 2020, creating the characteristic “first and second wave.”
As the virus spread, the number of patients requiring admission to the intensive care unit (ICU) increased rapidly, leading to massive challenges for the healthcare system 4 in general and ICUs in particular. COVID‐19 causes critical illness in 5% of cases 5 , 6 and one of the most common and life‐threatening complications of COVID‐19 is acute respiratory distress syndrome (ARDS). 7 ARDS is characterized by hypoxemic respiratory failure, pulmonary epithelial injury in the alveoli, and a systemic inflammatory response. 8 Patients with ARDS due to COVID‐19 generally require prolonged invasive mechanical ventilation (IMV) and are, therefore, often considered for tracheostomy. 4
The predominant indication for tracheostomy in patients with ARDS due to COVID‐19 is to facilitate prolonged IMV and aid when difficult weaning is anticipated. 9 Tracheostomy is associated with a lower requirement for sedatives and improved patient comfort, as well as optimized clearance of airway secretions. It facilitates oral care and reduces the risk of ventilator‐associated pneumonia and aspiration during weaning, while avoiding complications of long‐term oral intubation, such as tracheal stenosis. 10 , 11 In addition, tracheostomy tubes provide less need for breathing by bypassing the mouth and pharynx, thus creating less airway dead space. 4 As patients can be discharged to intermediate care facilities before decannulation, tracheostomy could potentially decrease the time in the ICU 12 liberating much needed resources.
Tracheostomy can be performed using either open surgical or percutaneous techniques. Studies have not shown significant differences in outcomes between the methods used for tracheostomy in COVID‐19 patients. 13
Performing tracheostomy has several advantages over oral intubation in critically ill patients requiring mechanical ventilation. 14 However, the optimal timing of the procedure has not been determined. Data on “early” vs. “late” tracheostomy regarding outcomes such as mortality and length of ICU and hospital stay are conflicting, and no consensus has yet been reached. 15 As the clinical trajectory of COVID‐19 was unclear at the onset of the pandemic, the timing of tracheostomy was even more ambiguous in these patients. 16
As tracheostomy is an aerosol‐generating procedure, 17 it is considered to be associated with a high risk of viral transmission to healthcare personnel, based on experience from the severe acute respiratory syndrome epidemic in 2003. 18 Because of this, recommendations about being restrictive when performing tracheostomy in COVID‐19 patients emerged at the onset of the pandemic. 19 , 20 Experts in otolaryngology suggested that tracheostomy should be delayed when the patient is cleared of infection, unless an adequate airway cannot be maintained with oral intubation. 21 In June 2020, the American College of Chest physicians recommended that tracheostomy should be considered in COVID‐19 patients, although evidence on the optimal timing of the procedure is still lacking. 22
In our hospitals, the strategy of COVID‐19 tracheostomies performed in the ICU by staff with adequate (hat, gown, shield or goggles, and Filtering Face Piece class 3 face mask) aerosol protection, remained operational throughout the first 18 months of the pandemic.
With limited data on this population, healthcare providers were unable to determine whether the potential benefits of tracheostomy in COVID‐19 patients exceeded the risk of infection in healthcare workers. During the pandemic, liberating ICU resources in a time of scarcity and maintaining patient care at an optimum level was a delicate balancing act.
To date, there are limited data on the clinical course and outcomes of tracheostomy in patients with COVID‐19. In addition, little is known regarding the optimal timing of tracheostomy in these patients. The aim of this study was to compare early and late tracheostomy in patients with COVID‐19‐related ARDS with regard to the clinical course and rates of tracheostomy‐related complications.
2. METHODS
This was a multicenter, retrospective observational study conducted at Ryhov County Hospital, Eksjö Hospital, and Värnamo Hospital, three nonacademic rural hospitals in Jönköping County in southern Sweden.
2.1. Patient selection and methods
The study included all adult patients (≥18 years old) who were admitted to an ICU in Jönköping County between March 14, 2020 and March 13, 2021 (Figure 1). All patients included were diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, as confirmed by positive real‐time polymerase chain reaction (PCR; LightMix Modular Sarbecovirus SARS‐CoV‐2 [Tib Molbiol] and Lyophilized 1‐step RT‐PCR Polymerase Mix [Tib]) results using nasopharyngeal swabs. The final date for data cutoff was April 14, 2021. Patients who were treated in the ICU in Jönköping County but underwent tracheostomy elsewhere were excluded. For patients transferred from our hospitals to different regions after receiving tracheostomy, data were collected only for the time in Jönköping County.
Figure 1.
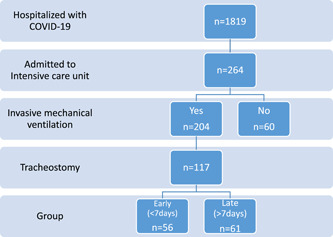
Patient selection flowchart
Patients who underwent tracheostomy were categorized into two groups based on the timing of the procedure. Those who were on mechanical ventilation for ≤7 days before surgery were included in the “early” group and those who were on mechanical ventilation for >7 days were included in the “late” group. 23 All tracheostomies were performed at the bedside in the ICU. Open surgical tracheostomy was performed in 116 (99.1%) cases.
Medical records from Cosmic™ and MetaVision™ were reviewed retrospectively by trained abstractors (ICU research unit nurses) to ensure accuracy. Data were inserted into a database (Microsoft® Excel for Windows) before data verification and statistical analysis
2.2. Main outcomes and measures
Patient demographics included age, sex, body mass index (BMI), the presence of comorbidities (cardiac disease, hypertension, diabetes, kidney/liver/lung disease, immunosuppression, neuromuscular disease, and active or previous cancer), as well as present or previous smoking habits and blood group. The primary outcome was the duration of IMV. The secondary outcomes included number of days on IMV before tracheostomy, time of decannulation, number of hours on mechanical ventilation in the prone position, ICU length of stay, all‐cause mortality (within 30 days of ICU admission), and complications associated with tracheostomy (hemorrhage, aspiration, displaced tracheal cannula, tracheal injury, failed surgery, accidental decannulation, stoma infection, perioperative hypoxemia, pneumothorax/pneumomediastinum/subcutaneous emphysema, fistulas, airway obstruction related to tracheostomy, and procedure‐related death).
2.3. Definitions
Comorbidities were defined by International Classification of Diseases‐10 codes in the medical records, that is, cardiovascular disease (I25 and/or I50), hypertension (I10), diabetes (E10/E11), asthma (J45), and chronic obstructive pulmonary disease (J44) in combination with active treatment. Immunosuppression was classified as systemic steroid treatment, radiation, or chemotherapy <6 months before hospital admission. 24 ARDS was defined using the criteria put forth by the Swedish Intensive Care Registry adopted from the Berlin definition. 24 Kidney function was defined using the “Kidney Disease: Improving Global Outcomes classification of chronic kidney disease” based on the glomerular filtration rate ml/min/1.73 m2.
Complications associated with tracheostomy were defined as events that had a potential negative impact on the patients' clinical course and thus required intervention. All events occurring from the time of the procedure to the time of decannulation were included. The definitions of the complications are outlined in Table 1.
Table 1.
Definitions of tracheostomy‐related complications
| Hemorrhage | In tracheal stoma, needing manual or pharmacological intervention |
|---|---|
| Displaced tracheal cannula | Displacement requiring manual repositioning |
| Aspiration | Visualization of gastric contents in the airway or clinical indications of aspiration |
| Tracheal injury | Lacerations or wounds related to the procedure or from chafing by the tracheal cannula |
| Failed surgery | Discontinuation of the procedure due to complications or unforeseen events |
| Pneumothorax/pneumomediastinum/subcutaneous emphysema | Verified by chest CT or clinical examination |
| Airway obstruction | Causing severe dyspnea, needing physician attention |
| Accidental decannulation | Tracheal cannula dislodgment |
| Stoma infection | Culture verified infection resulting in change in antibiotics |
| Fistula | Tracheoarterial or tracheoesophageal fistula |
Abbreviation: CT, computed tomography.
2.4. Ethical considerations
This study was approved by the Swedish Ethical Review Authority on August 26, 2020 (Dnr 2020‐02758). Patient consent was waived due to the retrospective nature of the study.
2.5. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics (version 27.0; IBM Corp.). Data are presented as descriptive statistics, with categorical variables reported as frequencies (percentages). The Shapiro–Wilk test was used to assess normality of data distribution. Nonparametric and parametric continuous variables are presented as medians (interquartile ranges [IQRs] and mean (SD), respectively. The differences between the groups were tested using the χ 2 test, the Mann–Whitney U test, Fisher's exact test, or Student's t test, as appropriate. All tests were two‐sided and statistical significance was set at p < 0.05.
3. RESULTS
3.1. Patient characteristics
Between March 14, 2020 and March 13, 2021, 264 patients with COVID‐19 were admitted to the ICU in 3 hospitals in Jönköping County, Sweden. Of the 204 patients requiring IMV, 117 (57%) underwent tracheostomy (Figure 1). The baseline characteristics are shown in Table 2. The baseline characteristics were similar between the early and late tracheostomy groups, with the exception that immunosuppressant treatment before ICU admission was more common in the late tracheostomy group (p = 0.026). In both the groups, the patients were predominantly men, with a median age of 66 years. Hypertension was the most frequent comorbidity (55%), followed by type 2 diabetes (28%). In total, 45% of the patients had a BMI of ≥30, with a significantly higher BMI (p = 0.025) among patients in the late tracheostomy group.
Table 2.
Baseline demographic characteristics
| Early tracheostomy (n = 56) | Late tracheostomy (n = 61) | Total (n = 117) | |
|---|---|---|---|
| Age, median (min–max) | 67 (22–87) | 65 (18–83) | 66 (18–87) |
| Gender, n (%) | |||
| Male | 46 (82) | 44 (72) | 90 (77) |
| Female | 10 (18) | 17 (28) | 27 (23) |
| Cardiovascular disease, n (%) | 9 (16) | 15 (25) | 24 (21) |
| Hypertension, n (%) | 27 (48) | 37 (61) | 64 (55) |
| Diabetes, n (%) | |||
| Type 1 | 2 (4) | 2 (3) | 4 (3) |
| Type 2 | 13 (23) | 20 (33) | 33 (28) |
| Liver cirrhosis, n (%) | 0 (0) | 1 (2) | 1 (1) |
| Neuromuscular disease, n (%) | 3 (6) | 2 (3) | 5 (4) |
| Immunosuppression, n (%) | 3 (6) | 12 (20) | 15 (13) |
| Asthma, n (%) | 6 (11) | 8 (13) | 14 (12) |
| COPD, n (%) | 6 (11) | 5 (8) | 11 (9) |
| Other pulmonary disease, n (%) | 7 (13) | 9 (15) | 16 (14) |
| Cancer, n (%) | |||
| Active | 4 (7) | 1 (2) | 5 (4) |
| Previous | 5 (9) | 1 (2) | 6 (5) |
| Kidney function, n (%) | |||
| KDIGO 1 | 27 (48) | 22 (36) | 49 (42) |
| KDIGO 2 | 29 (52) | 37 (60) | 66 (56) |
| KDIGO 3 | 0 (0) | 0 (0) | 0 (0) |
| KDIGO 4 | 0 (0) | 2 (3) | 2 (2) |
| KDIGO 5 | 0 (0) | 0 (0) | 0 (0) |
| Blood type, n (%) | |||
| 0 | 19 (34) | 25 (41) | 44 (38) |
| A | 29 (52) | 24 (39) | 53 (45) |
| B | 2 (4) | 11 (18) | 13 (11) |
| AB | 3 (6) | 1 (2) | 4 (3) |
| Active/previous smoking, n (%) | 19 (34) | 21 (34) | 40 (34) |
| BMI kg/m2, median (IQR) | 28 (26–33) | 31 (28–35) | 30 (27–34) |
| BMI ≥ 30 kg/m2, n (%) | 21 (38) | 32 (53) | 53 (45) |
| SAPS3, median (IQR) | 60 (49–63) | 58 (52–63) | 59 (51–63) |
| High‐dose prophylactic LMWH (%) | 48 (86) | 38 (62) | 86 (74) |
| ARDS grade | |||
| Mild | 2 (2) | 0 (0) | 2 (2) |
| Moderate | 44 (38) | 41 (35) | 85 (73) |
| Severe | 10 (9) | 20 (17) | 30 (30) |
| P/F‐ratio | |||
| 12 h postintubation | 195 (21,158–22,530) | 173 (135–225) | 180 (143–225) |
| 24 h postintubation | 188 (150–225) | 173 (128–218) | 188 (135–145) |
Note: Clinical characteristics of the study population, in total and divided by early and late tracheotomy. Categorical variables are reported as frequencies (%) and continuous variables as medians (IQR).
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; High‐dose LMWH, ≥9000 units/day low molecular weight heparin; SAPS3, simplified acute physiology score 3; P/F ratio, the ratio between partial pressure (mmHg) of oxygen in arterial blood over the fraction of inspired oxygen.
One patient remained on mechanical ventilation at the time of the final data cutoff. Six patients had no documented date of decannulation.
3.2. Tracheostomy outcomes
Compared with those who received late tracheostomy, patients who received early tracheostomy had a shorter median ICU stay, with an absolute difference of 8 days (p = 0.002). Early tracheostomy was also associated with fewer days of IMV (p = 0.001) than those in late tracheostomy (Table 3). There was no difference in the time from tracheostomy to weaning from mechanical ventilation or time of decannulation. In both the early and late tracheostomy groups, there were six deaths (10% and 11%, respectively; p > 0.99). The median durations from intubation to tracheostomy were 5 and 10 days in the early and late tracheostomy groups, respectively. The median time in the prone position during ICU stay was 32 h in the early tracheostomy group and 56 h in the late tracheostomy group.
Table 3.
Outcomes
| Early tracheotomy (≤7 days) | Late tracheotomy (>7 days) | p | |
|---|---|---|---|
| Days on ventilator (IQR) | 13 (9–20) | 20 (16–28) | 0.001 |
| Total ICU stay (IQR) | 16 (12–26) | 24 (19–34) | 0.002 |
| Days from tracheotomy to decannulation (IQR) | 13 (7–23) | 14 (8–22) | 0.914 |
| Days in IMV at tracheotomy, median (IQR) | 5 (4–6) | 10 (9–11) | – |
| Hours of IMV in prone position, median (IQR) | 32 (15–54) | 56 (21–99) | 0.052 |
| All‐cause mortality, 30 days, n (%) | 6 (11) | 6 (10) | >0.99 |
| Discharged to intermediate care before decannulation, n (%) | 30 (54) | 29 (48) | 0.46 |
Abbreviations: ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range.
Complications due to tracheostomy occurred in 42 patients (36%). The most frequent complication was stoma hemorrhage (requiring manual or pharmacological intervention), which occurred in 26 (22%) patients. Of the patients suffering from stomal hemorrhage, 21 (81%) were treated with high‐dose prophylactic low molecular weight heparin (LMWH) and 5 (19%) received low‐dose LMWH (p = 0.45). The total rate of complications was higher in the group receiving late tracheostomy; however, there were no differences between groups in terms of each complication by itself. A summary of the recorded complications is presented in Table 4.
Table 4.
Complications
| Early tracheotomy (≤7 days) | Late tracheotomy (>7 days) | p | |
|---|---|---|---|
| Hemorrhage | |||
| Perioperative | 0 (0) | 2 (3) | 0.5 |
| Early (≤2 days) | 6 (11) | 8 (13) | 0.78 |
| Late (>2 days) | 3 (5) | 7 (12) | 0.33 |
| Displaced tracheal cannula | 1 (2) | 1 (2) | >0.99 |
| Aspiration | |||
| Perioperative | 0 (0) | 0 (0) | – |
| Early (≤2 days) | 0 (0) | 0 (0) | – |
| Late (>2 days) | 0 (0) | 1 (2) | 0.92 |
| Tracheal injury | 1 (2) | 0 (0) | 0.67 |
| Failed surgery | 1 (2) | 0 | 0.48 |
| Accidental decannulation | 2 (4) | 1 (2) | 0.48 |
| Perioperative hypoxemia, POX O2 < 80% | 6 (11) | 9 (15) | 0.59 |
| Culture verified stoma infection | 1 (2) | 3 (5) | 0.62 |
| Pneumothorax/pneumomediastinum/sc emphysema | 9 (16) | 8 (13) | 0.79 |
| Fistula | 0 (0) | 0 (0) | – |
| Airway obstruction | 2 (4) | 6 (10) | 0.28 |
| Procedure‐related death | 0 (0) | 0 (0) | – |
| Any complication | 19 (34) | 23 (38) | 0.014 |
Note: Complications of tracheotomy, compared between early and late tracheotomy. Categorical variables are reported as frequencies and percentages, n (%).
Abbreviation: sc, subcutaneous.
4. DISCUSSION
Our results suggest that early tracheostomy (≤7 days) is associated with a shorter length of stay in the ICU and fewer days on IMV than those in late tracheostomy (>7 days). These findings illustrate the potential clinical benefits, including easier weaning from mechanical ventilation and performing tracheostomy earlier than what was recommended by international guidelines in the initial phase of the pandemic. Our results are in agreement with those of other studies. 16 , 25 However, the selection bias in the late tracheostomy group should be considered. Patients in the late group possibly had increased complexity in their clinical course compared to that in patients in the early group. This is indicated by patients in the late tracheostomy group having a higher incidence of immunosuppressant treatment (deduced from the medical history) along with requiring a longer time in prone position compared to the patients in the early tracheostomy group.
The BMI was significantly higher in patients who received late tracheostomy than in those who received early tracheostomy, with 53% of patients being obese (BMI ≥ 30) in the late group. As obesity is a risk factor for increased severity of COVID‐19, 25 this may have impacted the timing of tracheostomy being considered safe and profitable for the patient. Prone positioning has been shown to improve outcomes in patients with severe ARDS 26 and it may be more difficult to manage tracheostomy in obese patients, while in the prone position. Hence, obesity may have influenced decisions to postpone tracheostomy with the ambition to successfully extubate the patient or until prone positioning was no longer needed. In addition, open tracheostomy on obese, critically ill patients is associated with an increased risk of severe complications. 27
Comparison of all‐cause mortality 30 days after initial ICU admission showed no advantages for early or late tracheostomy. These findings are similar to those of other studies, including the landmark TracMan trial. 28 , 29 However, the low overall mortality rate in this study compared with that in other studies on tracheostomy in COVID‐19 patients limits comparativeness, as a small sample may influence statistical analysis.
In this study, clinically relevant complications due to tracheostomy occurred in 42 (36%) patients, compared with complication rates of 4%–22% reported in other studies. 4 , 28 , 30 , 31 When assessed as a composite variable, the total complication rates in this study somewhat exceed those in similar studies. However, the rates of each complication by itself are comparable to those of other studies. This indicates a variation in the definition of complications between studies, making comparison difficult. The difficulty in comparison is further aggravated by divergence in the tracheostomy technique (i.e., open vs. percutaneous) used due to local traditions and preferences between centers.
The most frequent complication in our patient group was hemorrhage in the tracheal stoma, appearing in 26 cases (22%). This was expected, as hemorrhage is reported to be one of the most common complications of tracheostomy in several studies. Due to the thrombogenic nature of COVID‐19, higher than usual doses of prophylactic LMWH are commonly used. Although not statistically significant, 81% of the patients suffering a stoma hemorrhage had high‐dose LMWH treatment. No major bleeding due to tracheostomy was observed. Complications, when assessed as composite variable, occurred more frequently in the group receiving late tracheostomy. However, when the specific complications were compared separately, no significant differences were observed between the groups (Table 4).
The decision to perform tracheostomy was made at the discretion of the consultant intensive care physician at each hospital. As ICU beds rapidly filled up and the supply of sedatives declined, the threshold for performing early tracheostomy was low. This approach was retained during the “second wave” of the pandemic and there was no difference in the time until tracheostomy between the first and second waves. The procedure was performed in collaboration among anaesthesiologists, otolaryngologists, and general surgeons. All but one tracheostomy procedure was performed with an open method, as this technique could allocate workload among specialties. Guidelines published in May 2020 also suggested that open tracheostomy generated less aerosols compared with percutaneous tracheostomy, thereby reducing the potential risk of viral transmission to healthcare workers. 32
There was no difference between the groups in the time from tracheostomy to liberation from mechanical ventilation, suggesting that the reduction in ventilator time is dependent on the shortening time from intubation to tracheostomy. These results favor an earlier approach, confirming the findings of similar studies. On the contrary, others continue to recommend a more conservative approach, which includes waiting for as long as 21 days before performing tracheostomy in COVID‐19 patients. 33 , 34 With no clear consensus, the decision regarding patient selection and timing of tracheostomy should be carefully evaluated and should not be made merely based on the time elapsed since intubation. As the course of this disease progresses, we will continue to learn more about the treatment strategies.
This study had several strengths. We included all eligible patients during the first year of the pandemic in our region and no patients were lost to follow‐up. Data were collected by several trained abstractors, and several key variables were collected automatically and prospectively by the same patient data management system in all participating hospitals. This study also had several limitations. First, due to its retrospective nature and lack of randomization between early and late tracheostomy, the risk of bias was high. Second, one patient remained in the ICU, and six patients had not been decannulated at the time of data cut‐off, introducing some uncertainty regarding the length of stay in the ICU, duration of mechanical ventilation, and number of days until decannulation. Third, the decision to perform tracheostomy was made at the discretion of the ICU consultant, which could include treatment heterogeneity between different physicians and hospitals.
5. CONCLUSIONS
This study showed that early tracheostomy is feasible and associated with shorter time on IMV and shorter ICU length of stay, indicating a possible clinical benefit in treating critically ill COVID‐19 patients. However, our findings need to be verified through randomized controlled trials. Complications, when assessed as composite variable, occurred more frequently in the group receiving late tracheostomy.
AUTHOR CONTRIBUTIONS
Anna Hansson: formal analysis; investigation; project administration; writing–original draft; writing–review and editing. Ola Sunnergren: conceptualization; formal analysis; investigation; methodology; project administration; supervision; writing–review and editing. Anneli Hammarskjöld: data curation; writing–review and editing. Catarina Alkemark: data curation; writing–review and editing. Knut Taxbro: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; validation; visualization; writing–original draft; writing–review and editing.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Swedish Ethical Review Authority on August 26, 2020 (Dnr 2020‐02758). Patient consent was waived due to the retrospective nature of the study.
TRANSPARENCY STATEMENT
The corresponding author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
We would like to express our gratitude to the staff of Jönköping County involved in caring for the COVID‐19 patients. We would also like to acknowledge S. Wennerholm and R. Svensson for their meticulous work in collecting data. This study was supported by Futurum, the Academy for Healthcare, Jönköping County Council, Jönköping, Sweden.
Hansson A, Sunnergren O, Hammarskjöld A, Alkemark C, Taxbro K. Characteristics, complications, and a comparison between early and late tracheostomy: a retrospective observational study on tracheostomy in patients with COVID‐19‐related acute respiratory distress syndrome. Health Sci Rep. 2022;5:e595. 10.1002/hsr2.595
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID‐19 patients: a meta‐analysis. J Med Virol. 2020;92(10):1902‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karadag E. Increase in COVID‐19 cases and case‐fatality and case‐recovery rates in Europe: a cross‐temporal meta‐analysis. J Med Virol. 2020;92(9):1511‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . 2021. Weekly operational update on COVID‐19. Available from https://www.who.int/publications/m/item/weekly-operational-update-on-covid-10---3-may-2021
- 4. Tang Y, Wu Y, Zhu F, et al. Tracheostomy in 80 COVID‐19 patients: a multicenter, retrospective, observational study. Front Med. 2020;7:615845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martínez‐Téllez E, Dotú CO, Trujillo‐Reyes JC, et al. Tracheotomy in patients COVID‐19: a necessary high risk procedure. Two center experience. Arch Bronconeumol. 2020;56(10):673‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tornari C, Surda P, Takhar A, et al. Tracheostomy, ventilatory wean, and decannulation in COVID‐19 patients. Eur Arch Otorhinolaryngol. 2020;278:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li X, Ma X. Acute respiratory failure in COVID‐19: is it "typical" ARDS? Crit Care. 2020;24(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Courtney A, Lignos L, Ward PA, Vizcaychipi MP. Surgical tracheostomy outcomes in COVID‐19‐positive patients. OTO Open. 2021;5(1):2473974x20984998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiba T, Ghazizadeh S, Chhetri, St John M, Long JD. Tracheostomy considerations during the COVID‐19 pandemic. OTO Open. 2020;4(2):2473974x20922528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nseir S, Di Pompeo C, Jozefowicz E, et al. Relationship between tracheotomy and ventilator‐associated pneumonia: a case control study. Eur Respir J. 2007;30(2):314‐320. [DOI] [PubMed] [Google Scholar]
- 12. Mattioli F, Fermi M, Ghirelli M, et al. Tracheostomy in the COVID‐19 pandemic. Eur Arch Otorhinolaryngol. 2020;277(7):2133‐2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Long SM, Chern A, Feit NZ, et al. Percutaneous and open tracheostomy in patients with COVID‐19: comparison and outcomes of an institutional series in New York City. Ann Surg. 2021;273(3):403‐409. [DOI] [PubMed] [Google Scholar]
- 14. Durbin CG, Jr. , Perkins MP, Moores LK. Should tracheostomy be performed as early as 72 hours in patients requiring prolonged mechanical ventilation? Respir Care. 2010;55(1):76‐87. [PubMed] [Google Scholar]
- 15. Wang R, Pan C, Wang, Xu F, Jiang S, Li MX. The impact of tracheotomy timing in critically ill patients undergoing mechanical ventilation: A meta‐analysis of randomized controlled clinical trials with trial sequential analysis. Heart Lung. 2019;48(1):46‐54. [DOI] [PubMed] [Google Scholar]
- 16. Kwak PE, Connors JR, Benedict PA, et al. Early outcomes from early tracheostomy for patients with COVID‐19. JAMA Otolaryngol Head Neck Surg. 2021;147(3):239‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei WI, Tuen HH, Ng, Ng RW, Lam LKRW. Safe tracheostomy for patients with severe acute respiratory syndrome. Laryngoscope. 2003;113(10):1777‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID‐19 pandemic. JAMA Otolaryngol Head Neck Surg. 2020;146(6):579‐584. [DOI] [PubMed] [Google Scholar]
- 20. Smith D, Montagne J, Raices M, et al. Tracheostomy in the intensive care unit: guidelines during COVID‐19 worldwide pandemic. Am J Otolaryngol. 2020;41(5):102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sommer DD, Engels PT, Weitzel EK, et al. Recommendations from the CSO‐HNS taskforce on performance of tracheotomy during the COVID‐19 pandemic. J Otolaryngol Head Neck Surg. 2020;49(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamb CR, Desai NR, Angel L, et al. Use of tracheostomy during the COVID‐19 pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology Program Directors Expert Panel Report. Chest. 2020;158(4):1499‐1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adly A, Youssef TA, El‐Begermy MM, Younis HM. Timing of tracheostomy in patients with prolonged endotracheal intubation: a systematic review. Eur Arch Otorhinolaryngol. 2018;275:679‐690. 10.1007/s00405-017-4838-7 [DOI] [PubMed] [Google Scholar]
- 24. Svenska intensivvårdsregistret . 2016. Riskjusteringsmodeller inom svensk intensivvård (cited May 4, 2021). 17.0. Available from https://www.icuregswe.org/globalassets/riktlinjer/riskjustering.pdf
- 25. Queen Elizabeth Hospital Birmingham COVID Airway Team . Safety and 30‐day outcomes of tracheostomy for COVID‐19: a prospective observational cohort study. Br J Anaesth. 2020;125(6):872‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soeroto AY, Soetedjo NN, Purwiga A, et al. Effect of increased BMI and obesity on the outcome of COVID‐19 adult patients: a systematic review and meta‐analysis. Diabetes Metab Syndr. 2020;14(6):1897‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Solh AA, Jaafar W. A comparative study of the complications of surgical tracheostomy in morbidly obese critically ill patients. Crit Care. 2007;11(1):R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young D, Harrison DA, Cuthbertson BH, Rowan K, TracMan Collaborators . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121‐2129. [DOI] [PubMed] [Google Scholar]
- 29. Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically ill patients: a meta‐analysis. JAMA Otolaryngol Head Neck Surg. 2021;147:450‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Avilés‐Jurado FX, Prieto‐Alhambra D, González‐Sánchez N, et al. Timing, complications, and safety of tracheotomy in critically ill patients with COVID‐19. JAMA Otolaryngol Head Neck Surg. 2020;147(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chao TN, Harbison SP, Braslow BM, et al. Outcomes after tracheostomy in COVID‐19 patients. Ann Surg. 2020;272(3):e181‐e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGrath BA, Brenner MJ, Warrillow SJ, et al. Tracheostomy in the COVID‐19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8(7):717‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Volo T, Stritoni P, Battel I, et al. Elective tracheostomy during COVID‐19 outbreak: to whom, when, how? Early experience from Venice, Italy. Eur Arch Otorhinolaryngol. 2021;278(3):781‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishio N, Hiramatsu M, Goto Y, et al. Surgical strategy and optimal timing of tracheostomy in patients with COVID‐19: early experiences in Japan. Auris Nasus Larynx. 2021;48(3):518‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


