Abstract
Plant malectin-like receptor kinases (MLRs), also known as Catharanthus roseus receptor-like kinase-1-like proteins, are well known for their functions in pollen tube reception and tip growth, cell wall integrity sensing, and hormonal responses. Recently, mounting evidence has indicated a critical role for MLRs in plant immunity. Here we focus on the emerging functions of MLRs in modulating the two-tiered immune system mediated by cell-surface-resident pattern recognition receptors (PRRs) and intracellular nucleotide-binding leucine-rich repeat receptors (NLRs). MLRs complex with PRRs and NLRs and regulate immune receptor complex formation and stability. Rapid alkalinization factor peptide ligands, LORELEI-like glycosylphosphatidylinositol-anchored proteins and cell-wall-associated leucine-rich repeat extensins coordinate with MLRs to orchestrate PRR- and NLR-mediated immunity. We discuss the common theme and unique features of MLR complexes concatenating different branches of plant immune signalling.
To survive during plant–pathogen warfare, plants have evolved a two-tiered immune system: pattern-triggered immunity (PTI) and effector-triggered immunity (ETI)1,2. PTI is triggered via the cell-surface-resident pattern recognition receptors (PRRs), including receptor kinases (RKs) or receptor-like kinases and receptor-like proteins, which sense the microorganism-/damage-associated molecular patterns (MAMPs/DAMPs) and confer resistance against a broad spectrum of pathogens3–5. Some adaptive pathogens successfully evade plant resistance by delivering effectors into host cells to interfere with PTI or plant physiology6. Intracellular nucleotide-binding leucine-rich repeat receptors (NLRs) sense the effectors directly or indirectly and trigger plant ETI, usually resulting in a more robust response than PTI and localized cell death as the hypersensitive response7,8. Although PTI and ETI are activated by different immune receptors accompanied by some specific early signalling events, PRR- and NLR-mediated signalling converges at multiple levels9,10. Recent findings indicate that PTI and ETI mutually potentiate each other and are jointly required to provide a robust defence response against pathogens11,12. In addition, a series of shared signalling events have been observed upon PTI and ETI activation (such as calcium influx, reactive oxygen species (ROS) burst, modulation of hormonal levels and transcriptional regulation of defence genes), though often with distinct spatio-temporal dynamics and strength13.
An RK typically consists of an ectodomain (ECD), a transmembrane domain and an intracellular kinase domain14. On the basis of the heterogeneity of their ECDs, RKs are classified into several subgroups, including leucine-rich repeat (LRR), lysin motif, lectin motif, cysteine-rich, epidermal-growth-factor-like and malectin-like14,15. Malectin-like RKs (MLRs)16, harbouring two tandem malectin‐like domains in their ECD, are also known as Catharanthus roseus receptor-like kinase-1-like kinases (CrRLK1Ls), as they were first characterized in Madagascar periwinkle (C. roseus)17. There are 17 MLR members in Arabidopsis with versatile functions in plant development, such as polarized growth, cell elongation, cell wall integrity (CWI) sensing and hormonal responses18–22. FERONIA (FER), the best-characterized member of the MLRs, controls a myriad of biological processes in different plant tissues, including root hair growth, female gametophytic control of pollen tube reception, plant hormone signalling and immunity23–25. ANXUR1 (ANX1) and ANX2, close homologues of FER, maintain pollen tube integrity and regulate male fertility during reproduction26–28. Buddha’s Paper Seal 1 (BUPS1) and BUPS2 interact with ANX1/ANX2 and regulate CWI during pollen tube growth29–31. MLRs were named according to Etruscan, Greek, Roman and Chinese mythologies. For instance, Feronia was named after an Etruscan goddess of fertility, and Anxur was the consort of Feronia; Buddha’s Paper Seal came from the Chinese myth ‘Journey to the West’. A group of secreted peptides known as rapid alkalinization factors (RALFs) bind to MLR ECDs as ligands to modulate MLR activation. Additionally, LORELEI-like glycosylphosphatidylinositol-anchored proteins (LLGs) and cell-wall-associated LRR extensin proteins (LRXs) play intertwined roles with MLRs in perceiving RALFs and regulating diverse physiological processes32. Emerging evidence supports the critical roles of RALF–MLRs, together with LLGs and LRXs, in plant immunity (Fig. 1). They regulate PTI and ETI at multiple steps, from modulating immune receptor complex activation and stability to regulating defence hormone signalling (Fig. 1). Consequently, cellular perturbation triggered by the activation of PRRs or NLRs during infections could be monitored by MLR complexes, which might contribute to the convergence of PTI and ETI. This Perspective will highlight and discuss the emerging functions of MLRs and the related proteins in plant immunity.
Fig. 1 |. The MLR–LLG–RALF–LRX module regulates plant immunity.
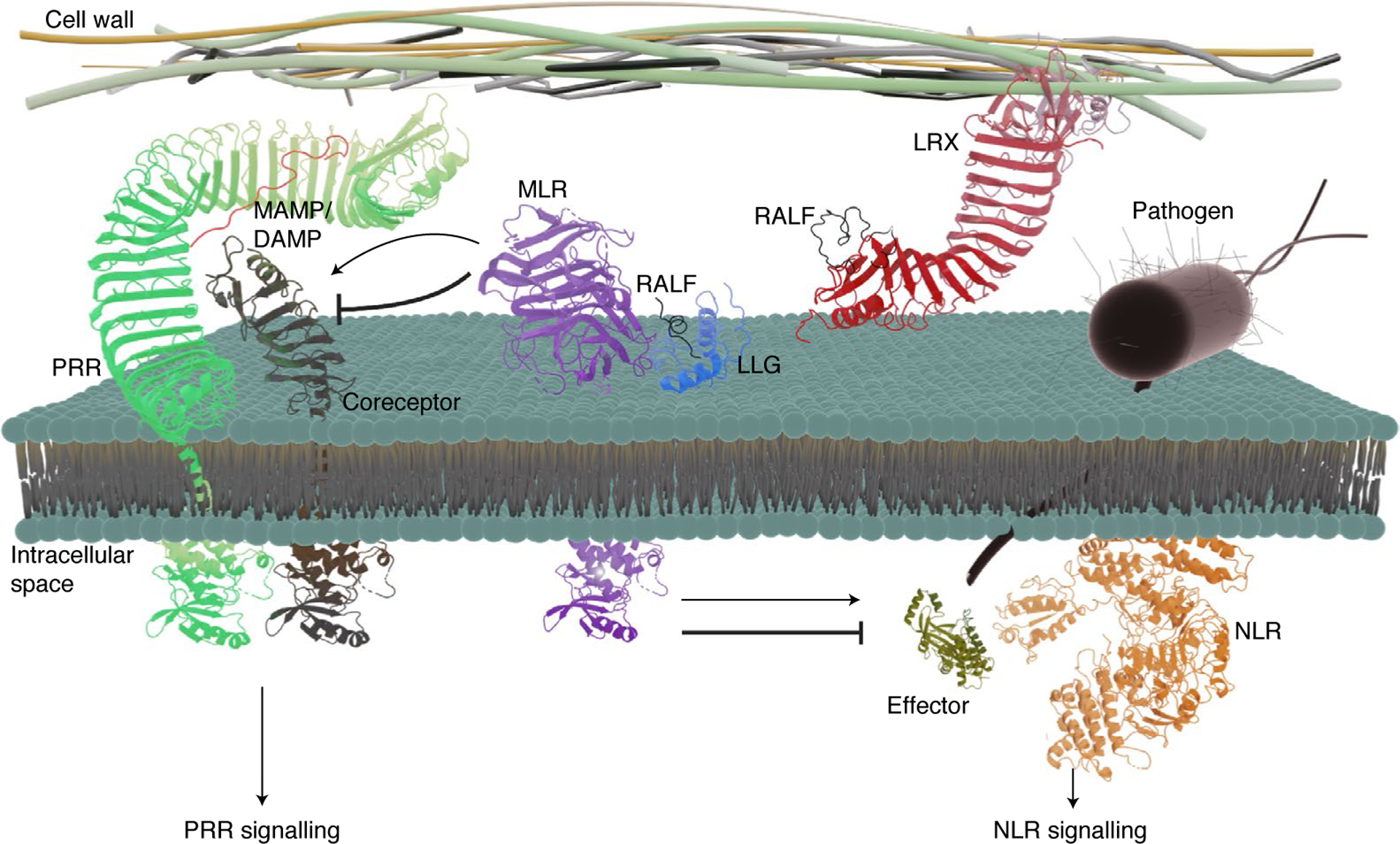
Following MAMP or DAMP recognition, PRR complexes with the coreceptor, followed by the activation of PRR-mediated immune signalling and PTI. To facilitate infection, pathogens deliver a suite of effector proteins into host cells through specialized structures. The effectors could be recognized directly or indirectly by NLRs, resulting in the activation of NLR-mediated immune signaling and ETI. Emerging evidence indicates that MLRs together with LLGs modulate PRR and NLR signalling at multiple steps. RALF peptides are the ligands of MLR–LLG modules and the cell-wall-binding LRX proteins. The extracellular domain of the MAMP/DAMP-induced PRR–coreceptor complex is represented by the protein structure of the flg22-induced FLS2–BAK1 ECD complex (PDB: 4MN8). The extracellular domain of MLR complexed with LLG and RALF is represented by the structure of the extracellular domain of FER in complex with LLG2 and RALF23 (PDB: 6A5E). The transmembrane helices of PRR and its coreceptor were added to depict the structure representation; however, they do not represent the actual structure of the transmembrane domains of these proteins. The tyrosine kinase domain of the c-Abl human protein (PDB: 2FO0) was used to represent the kinase domain of PRR and its coreceptor, as well as MLR. The LRR domain of LRX complexed with RALF is represented by the structure of LRX8 and RALF4 complex (PDB: 6QXP). The effector is represented by the structure of the effector AvrB (PDB: 1NH1). NLR is represented by the structure of the NLR ZAR1 (PDB: 6J6I). The molecular structures were visualized using PyMOL Molecular Graphics System, v.2.4 Schrödinger, LLC (http://www.pymol.org/). This figure was created using Blender.
MLRs regulate plant PRR-mediated immunity
The fer mutant, with pleiotropic growth defects, was initially reported to display enhanced resistance against powdery mildew infections, hinting at a yet elusive role of FER as a susceptibility factor for this biotrophic fungus33. Later, the fer mutant was found to be more susceptible to the bacterial pathogen Pseudomonas syringae pv. tomato DC3000, and FER was shown to positively regulate PTI-induced ROS production by facilitating the ligand-induced formation of PRR complexes, including ELONGATION FACTOR-Tu RECEPTOR (EFR)/FLAGELLIN SENSING 2 (FLS2) and their coreceptor BRASSINOSTEROID INSENSITIVE 1 (BRI1)-ASSOCIATED KINASE 1 (BAK1) (Fig. 2a)34. FER function in plant PTI is independent of its kinase activity and was proposed to function as a scaffold modulating PRR complex assembly35. The function of FER in plant PTI is inhibited by the perception of RALF23, a peptide released from its precursor PRORALF23 upon MAMP treatment34. Hence, MAMP perception induces RALF23 production, which is perceived by FER, consequently inhibiting FER function in scaffolding PRR complexes (Fig. 2a). In contrast, ANX1 and ANX2 negatively regulate plant PTI by interfering with MAMP-induced PRR complex formation36. ANX1 and ANX2 constitutively associate with FLS2; however, their association with BAK1 is enhanced upon the perception of the MAMP flagellin, which may compete for flagellin-induced FLS2–BAK1 complex assembly (Fig. 2b)36. It remains puzzling how FER and ANXs carry the opposite functions in plant PTI. Do they compete for the RALF ligands? The ligands of ANXs in plant immunity have not been identified. Pollen-tube-expressed RALF4 and RALF19, possibly competing with female-derived RALF34, bind to ANX1 and ANX2 and are required for ANX-mediated pollen tube integrity29. Are RALF4 and RALF19 also the ligands of ANXs in plant immunity? Alternatively, do ANXs compete with FER for binding to PRRs or vice versa? Comparing the binding affinity of ANXs and FER with PRR ECDs and identifying bona fide ligands of ANXs in leaves during PTI might provide insights into these questions.
Fig. 2 |. Arabidopsis MLRs regulate different aspects of plant immunity.
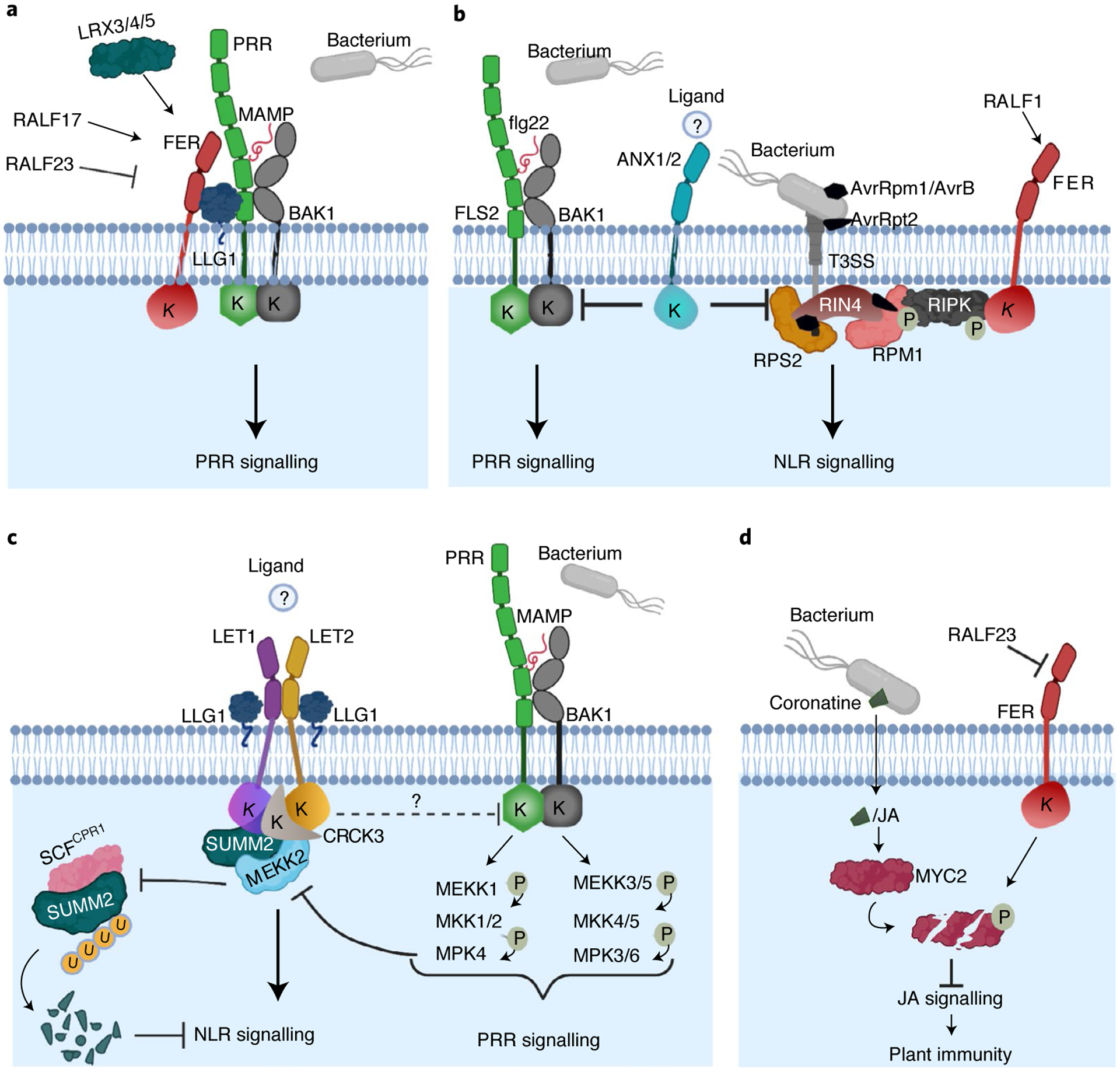
a, FER positively regulates PRR signalling. FER, together with LLG1, scaffolds the MAMP-induced PRR–BAK1 complex. This function is positively modulated upon the perception of RALF17 and negatively modulated by RALF23, and requires the cell-wall-binding LRX proteins. b, ANX1 and ANX2 negatively regulate PRR and NLR signalling. ANX1 and ANX2 interact with PRR FLS2–BAK1 and NLR RPS2 and RPM1 proteins. NLR signalling activation by RPS2 and RPM1 is mediated by modifications of RIN4 upon recognition of AvrRpt2 and AvrRpm1/AvrB effectors, respectively. ANX1 and ANX2 associate with RIPK, which phosphorylates RIN4 in the presence of AvrRpm1/AvrB. Effectors are translocated into the host cell through the bacterial type III secretion system (T3SS). The ligand of ANX1/ANX2 in plant immunity remains unknown. RALF1 triggers FER-mediated RIPK phosphorylation, which might play a role in the modulation of RPM1 signalling. c, MAMP-activated MEKK1–MKK1/2–MPK4 cascade regulates PRR signalling and SUMM2-mediated autoimmunity. MEKK2 scaffolds LET1 and SUMM2 for protein stability and complex assembly and counter-regulates SCFCPR1-mediated SUMM2 ubiquitylation and degradation. LET1 and LET2, together with LLG1, form a trimeric complex to regulate SUMM2 activation. It is unknown whether LET1 and LET2 can also regulate the function of PRRs. LET2 associates with CRCK3, which is proposed to be guarded by SUMM2 to monitor the integrity of the MEKK1–MKK1/2–MPK4 cascade. The activation of LET1 and LET2 could be modulated by an unidentified ligand. d, FER contributes to plant immunity by suppressing JA signalling through negative regulation of MYC2 stability. To promote infection, bacteria release the phytotoxin coronatine into the host, which mimics the action of the hormone JA and activates the transcription factor MYC2. FER phosphorylates and leads to the degradation of MYC2, thereby suppressing JA signalling and positively contributing to plant immunity; RALF23 inhibits FER action. A circled P indicates a phosphorylation event. A circled U indicates ubiquitin. K indicates a kinase domain. This figure was created with BioRender.
The altered immune responses in anx mutants were much more pronounced at the four-week-old stage than at the two-week-old stage, suggesting that the function of ANXs might depend on developmental stage and/or growth condition. The spatio-temporal expression and regulation of MLR–RALF interactions during reproduction are well documented23,29,37,38. Notably, immunity varies with age, and the ontogeny of FLS2-mediated immunity is correlated with the microRNA-regulated FLS2 transcript level during development39. It remains to be determined whether a similar mechanism regulates RALF–FER/ANX functions in plant immunity or whether RALF–FER/ANX ligand–receptor pairs are involved in the crosstalk between plant development and immunity.
MLR homologues in other plant species have also been implicated in plant immunity. Soybean Glycine max LESION MIMIC MUTANT 1 (GmLMM1), an MLR, interacts with GmFLS2 and GmBAK1 and negatively regulates PTI by suppressing flagellin-induced GmFLS2–GmBAK1 complex formation, resembling the role of Arabidopsis ANXs in modulating plant PTI40. Unlike Arabidopsis FER and ANXs, GmLMM1 constitutively interacts with GmBAK1, and ligand perception potentiates GmLMM1–FLS2 interaction40. Systematic gene expression and functional screen of rice FER-LIKE RECEPTORs (FLRs) indicate that different FLRs positively or negatively regulate basal resistance to rice blast fungus (Magnaporthe oryzae)41,42.
The glycosylphosphatidylinositol-anchored protein LORELEI and LLGs function as adaptors or coreceptors for FER, BUPSs and ANXs in regulating plant growth and reproduction43–45. LLGs directly interact with the extracellular juxtamembrane region of MLRs and assist in protein delivery from the endoplasmic reticulum to the plasma membrane (PM)45,46. Like FER, LLG1 modulates flagellin-induced FLS2–BAK1 complex assembly and is involved in RALF23-induced FER-dependent suppression of plant PTI47, pointing to the possibility that FER and LLG1 function together in regulating PRR complex formation. In addition, similar to FER and ANXs, LLG1 complexes with PRR, FLS2 and EFR constitutively but with the BAK1 coreceptor in a ligand-dependent manner48. Furthermore, LLG1 stabilizes FLS2 proteins and modulates the accumulation and ligand-induced degradation of FLS2 (ref.48). Thus, LLG1 not only regulates MLR homeostasis and signalling but also modulates LRR–RK abundance. It is possible that LLG1 regulation of LRR–RKs is bridged through FER and ANXs.
Although FER and LLG1 are important for MAMP-induced ROS production, the function of FER and LLG1 in other PTI early responses (such as the activation of mitogen-activated protein kinases (MAPKs) and induction of some early PTI marker genes) has not been established. Notably, many PTI marker genes exhibit increased expression levels in the fer-4 transcriptome49. Similarly, the llg1 mutant also seems to have increased expression of the PTI marker gene FLG22-INDUCED RECEPTOR-LIKE KINASE 1 (FRK1) and activation of MAPKs after MAMP flg22 peptide treatment48. It is puzzling how FER and LLG1 function in the same complex with PRRs but have different effects on various PRR-mediated responses. MAMP-induced ROS production is mainly mediated by receptor-like cytoplasmic kinase (RLCK)-regulated phosphorylation of NADPH oxidases50. It is possible that FER–LLG1-modulated PRR complex assembly might have a major effect on RLCK phosphorylation. Alternatively, FER and LLG1 may also regulate MAMP-induced ROS production through activating the plant Rho-like GTPase (RAC/ROP)–NADPH oxidase pathway, as they do in root hair development and pollination24,51.
MLRs regulate plant NLR-mediated immunity
Accumulating evidence also highlights MLRs as critical regulators in NLR-mediated immunity. ANX1 and ANX2 negatively regulate plant ETI by association with PM-resident NLR protein RESISTANCE TO PSEUDOMONAS SYRINGAE PV. MACULICOLA 1 (RPM1) and RESISTANCE TO PSEUDOMONAS SYRINGAE 2 (RPS2) (Fig. 2b)36. Interestingly, ANX1 attenuates RPS2-triggered cell death by promoting RPS2 degradation36. The stability of RPS2 is regulated by multiple E3 ligase-mediated ubiquitylation, including the F-box protein CONSTITUTIVE EXPRESSER OF PR GENES 1 (CPR1)-containing SKP1–CULLIN1–F-box (SCF) complex52,53, and RING-type SNC1-INFLUENCING PLANT E3 LIGASE REVERSE 1/2 (SNIPER1/2)54. It will be interesting to investigate whether ANX1/ANX2-mediated RPS2 degradation depends on E3 ligase SCFCPR1 and/or SNIPER1/2. In addition, ANX1 associates with RLCK RPM1-INDUCED PROTEIN KINASE (RIPK)36. Upon P. syringae effector AvrRpm1 or AvrB stimulation, RIPK phosphorylates RPM1-INTERACTING PROTEIN 4 (RIN4) to activate RPM1-mediated ETI responses55–57. FER also interacts with RIPK, and RALF1 triggers FER-mediated RIPK phosphorylation to regulate root growth58, suggesting a potential link of RALF1–FER signalling in RIPK-mediated RPM1-induced immunity (Fig. 2b).
Recently, compelling evidence has supported the positive role of MLRs in the activation of NLR-mediated autoimmunity46,59,60. The perception of MAMPs by PRRs activates two parallel MAPK modules, MKKK3/5–MKK4/5–MPK3/6 and MEKK1–MKK1/2–MPK4, in Arabidopsis (Fig. 2c)61–63. The disruption of the MEKK1–MKK1/2–MPK4 cascade leads to autoimmunity, which largely depends on the NLR protein SUPPRESSOR OF mkk1 mkk2 2 (SUMM2)64–68 and conditionally depends on NLR RPS6 (ref.69). Silencing MEKK1 by Agrobacterium-mediated virus-induced gene silencing (VIGS) triggers cell death and a dwarfism phenotype70. A VIGS-based interference RNA (RNAi) screen that silenced MEKK1 in the homozygous Arabidopsis T-DNA insertion mutant library identified a mutant of MLR, lethality suppressor of mekk1 1 (letum1 or LET1), as a suppressor of the cell death caused by silencing MEKK1 (ref.59). Letum, also known as Mors, is the personification of death in Roman mythology. A systematic screen of the MLR family members identified another MLR, LET2 (also named MEDOS1), that functions additively with LET1 in regulating SUMM2-mediated autoimmunity46,60. LET2 complexes with LET1 and promotes LET1 phosphorylation, revealing a phosphoregulation loop between different MLRs46. Heteromeric complex formation among different MLRs has also been observed for BUPSs–ANXs and for HERCULES RECEPTOR KINASE 1 (HERK1)/ANJEA (ANJ)–FER in regulating pollen tube integrity and reception29,30,71. The biological function and relevance of multimeric MLR complex formation have yet to be elucidated. It is possible that some MLRs in the complex function as a scaffold, such as FER, to modulate other MLR stability or localization. Alternatively, some MLRs may regulate other MLR activities, such as through phosphorylation in the case of LET1 and LET2. LLGs and LORELEI are part of the multimeric MLR protein complexes43–45. LLG1 interacts with LET1/LET2 and regulates SUMM2 activation (Fig. 2c)46.
MEKK2, a MAPK kinase kinase, and CALMODULIN-BINDING RECEPTOR-LIKE CYTOPLASMIC KINASE 3 (CRCK3), also regulate SUMM2 activation72–74. LET1 and LET2 form a multimeric complex with SUMM2, MEKK2 and CRCK3 (refs.46,59,60). Although annotated as a kinase, MEKK2 does not have detectable kinase activity, and its kinase-inactive mutant triggers comparable autoimmunity with wild-type MEKK2 (ref.70,75). MEKK2 acts as a scaffold and regulates SUMM2 homeostasis by counter-regulating SCFCPR1-mediated SUMM2 ubiquitylation and degradation (Fig. 2c)59. MEKK2 interacts with both MLR LET1 and NLR SUMM2, and it stabilizes LET1 and SUMM2 for immune activation59. A critical knowledge gap is how LET1 and LET2 activate SUMM2. Notably, LET2 phosphorylates CRCK3 (ref.60). It is possible that a sequential phosphorylation module consisting of the LET2–LET1–CRCK3 complex is guarded or sensed by SUMM2 for the activation of SUMM2-mediated immunity. Alternatively, the LET2–LET1–CRCK3 kinase cascade phosphorylates SUMM2, thereby leading to SUMM2 subcellular relocalization or conformational changes, such as disrupting SUMM2 intramolecular interaction for activation. The association of SUMM2 with the MEKK2 pseudokinase and the LET2–LET1–CRCK3 kinase module echoes the composition of the NLR HOPZ-ACTIVATED RESISTANCE 1 (ZAR1) resistosome. Structural data of the ZAR1 resistosome have revealed that the pseudokinase RESISTANCE-RELATED KINASE 1, RLCK avrP-phB SENSITIVE 1-LIKE 2 (PBL2) and NLR ZAR1 assemble into a pathogen sensor complex, and pathogen effector-triggered modification on PBL2 switches on ZAR1 activation by forming a wheel-like pentamer with an N-terminal funnel-shaped structure associated with the PM76,77. It is tempting to speculate that SUMM2 activation might share a similar feature with the ZAR1 resistosome, and alteration of the MEKK1–MKK1/2–MPK4 cascade might be sensed by the SUMM2 resistosome consisting of the MEKK2 pseudokinase, LET2–LET1–CRCK3 kinase cascade, and NLR SUMM2.
Loss-of-function mutants of soybean MLR GmLMM1 display an autoimmunity-related lesion mimic phenotype40. The lesion mimic mutants are usually caused by the activation of NLRs78. An unknown NLR might be activated in the Gmlmm1 mutants, which is otherwise suppressed by GmLMM1 in wild-type plants. Thus, similar to ANX1 and ANX2, GmLMM1 might negatively regulate both PRR- and NLR-mediated immunity.
MLRs regulate plant defence hormone-mediated immunity
Plant hormones, including salicylic acid (SA), jasmonic acid (JA), ethylene (ET), brassinosteroids (BRs), abscisic acid (ABA) and auxin, play essential and intertwined roles in plant immune responses79,80. JA and ET are crucial in responses to necrotrophic pathogens and herbivores, whereas SA mediates resistance against biotrophic and hemibiotrophic pathogens79,80. The effects of MLRs in different hormone signalling pathways have been reported24,25 (Fig. 3). For example, FER negatively regulates JA, ET and ABA responses but positively regulates BR and auxin signalling24,25,49,81–83.
Fig. 3 |. Multiple functions of Arabidopsis MLRs in PRR- and NLR-mediated immunity, hormone signalling and CWI sensing, culminating in a balanced growth–defence trade-off.
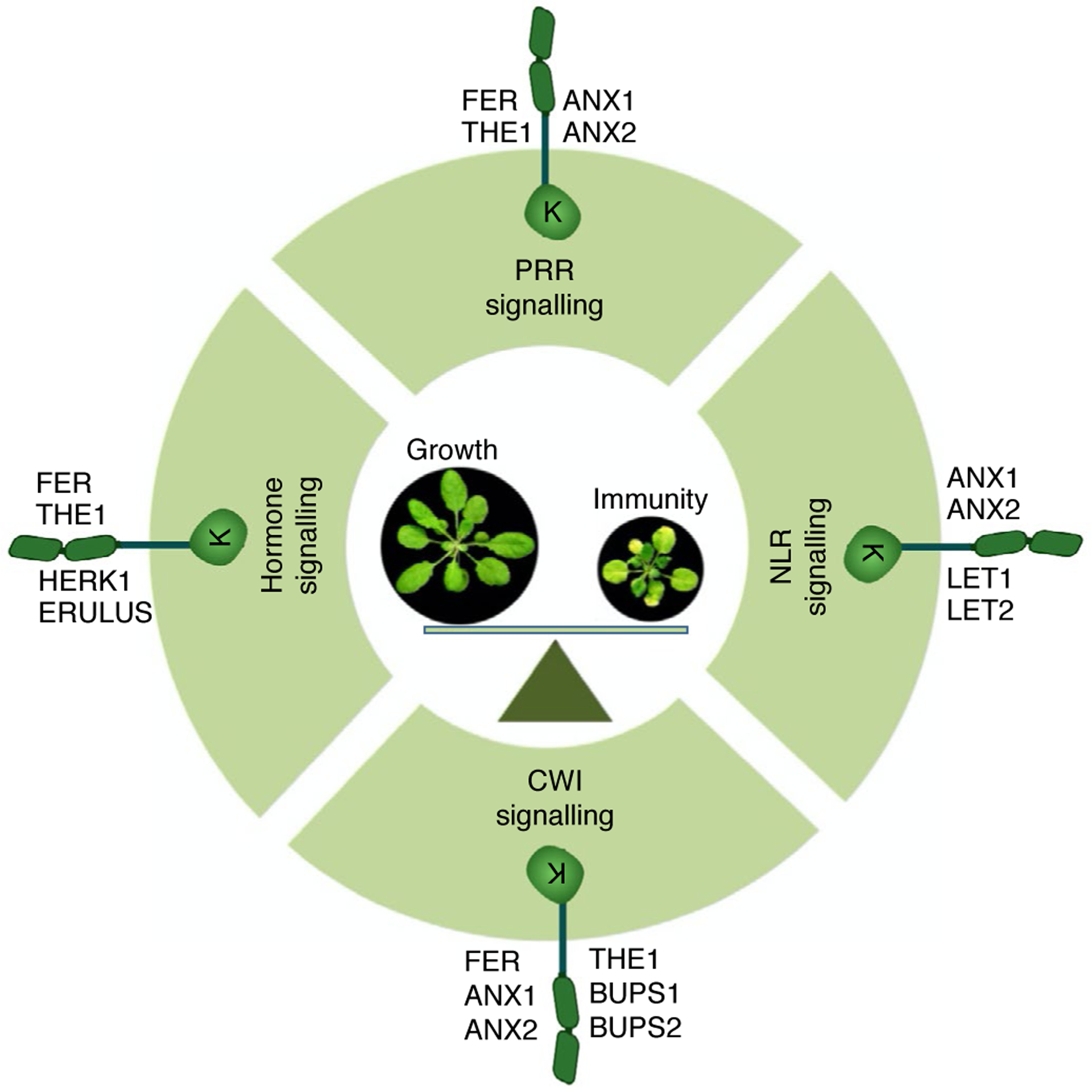
Different MLR family members are implicated in PRR, NLR, CWI and hormone signalling processes that potentially orchestrate the growth–defence trade-off in plants. This figure was created with BioRender.
Besides positive regulation of plant immunity to P. syringae by functioning as a PRR scaffold, FER positively regulates plant immunity to this hemibiotrophic bacterium by downregulating JA signalling49. The transcription factor MYC2 is a crucial regulator of JA signalling that often antagonizes SA accumulation and signalling84. FER directly phosphorylates MYC2, leading to MYC2 degradation49. RALF23 inhibits FER’s negative regulation of MYC2 stability and promotes JA signalling, favouring bacterial infection (Fig. 2d)49. This study establishes a direct link between an MLR and a transcription factor in regulating gene expression. As a critical transcription factor, MYC2 interacts with multiple regulators in the nucleus, and its stability is modulated by several E3 ligase-mediated ubiquitylation85. It will be interesting to determine the subcellular compartmentation of FER–MYC2 interaction and the mode of action of FER phosphorylation-mediated MYC2 degradation. Notably, the activation of JA signalling in the fer mutants occurs only in shoots, but not roots86.
The MLR THESEUS1 (THE1) positively regulates plant defence against the necrotrophic fungal pathogen Botrytis cinerea87. THE1 interacts with GUANINE EXCHANGE FACTOR 4, which might relay THE1 signalling in response to B. cinerea. Overexpression of THE1 misregulates the expression of a large number of hormone-responsive genes regulated by JA, ET, SA, ABA and BR upon B. cinerea infection, suggesting that THE1 functions in plant defence partially through the modulation of plant hormones87. THE1 acts as a sensor of CWI during cell elongation (Fig. 3)88, hinting that THE1 might sense cell wall modifications upon B. cinerea infection and transduce the defence signalling through GUANINE EXCHANGE FACTOR 4 (ref.87).
Pathogen-encoded RALF mimics modulate plant MLR-mediated immune responses
Molecular mimicry is a strategy employed by pathogens to colonize their hosts, in which pathogen molecules mechanistically mimic the functions of host components and modulate host responses to promote pathogenicity and fitness89. Homologues of plant RALFs were identified as widely distributed across phylogenetically distant fungi, including some economically important phytopathogens, such as Fusarium oxysporum and Fusarium graminearum90–92. The RALF-like genes are also present in multiple species of root-knot nematodes, including Meloidogyne incognita93. Moreover, the RALF-like genes and genes carrying the RALF motif were predicted in some bacterial Escherichia coli strains and plant-pathogenic Actinobacteria90,91. The wide distribution of RALF-like sequences in different microorganisms across kingdoms suggests their importance in parasitism and association with the hosts.
Some pathogen RALF-like peptides could mimic plant RALFs and modulate FER-mediated responses, favouring the pathogen infection process (Fig. 4). For instance, the root-infecting fungus F. oxysporum secretes a functional RALF mimic (F-RALF) that, like plant homologues, induces extracellular alkalinization, which might stimulate the phosphorylation and activation of F. oxysporum MAPK signalling to promote pathogen virulence90. Furthermore, F-RALF is crucial for suppressing multiple defence responses in tomatoes and promoting F. oxysporum infection by directly targeting FER90. Conversely, the Arabidopsis fer mutant exhibited enhanced resistance against F. oxysporum and compromised F-RALF-triggered responses90. Likewise, the root-knot nematode M. incognita contains two RALF-like genes (MiRALF1 and MiRALF3), which are upregulated at the parasitic stage and required for its parasitism93. MiRALF1 and MiRALF3 mimic host RALFs and bind FER, thereby manipulating FER-mediated immune responses and plant growth to promote M. incognita parasitism93. In addition, like Arabidopsis RALF1, MiRALF1 and MiRALF3 reduce the protein level of MYC2, a key regulator of JA signalling, suggesting that M. incognita manipulates JA signalling during the parasitism process93. The nematode parasitism mediated by RALF-like peptides via FER seems to be a conserved mechanism in plants, as the rice homologue of FER, FLR1, is also required for M. incognita infection93.
Fig. 4 |. Pathogen RALF peptides mimic host RALFs and modulate FER-mediated responses during infections.
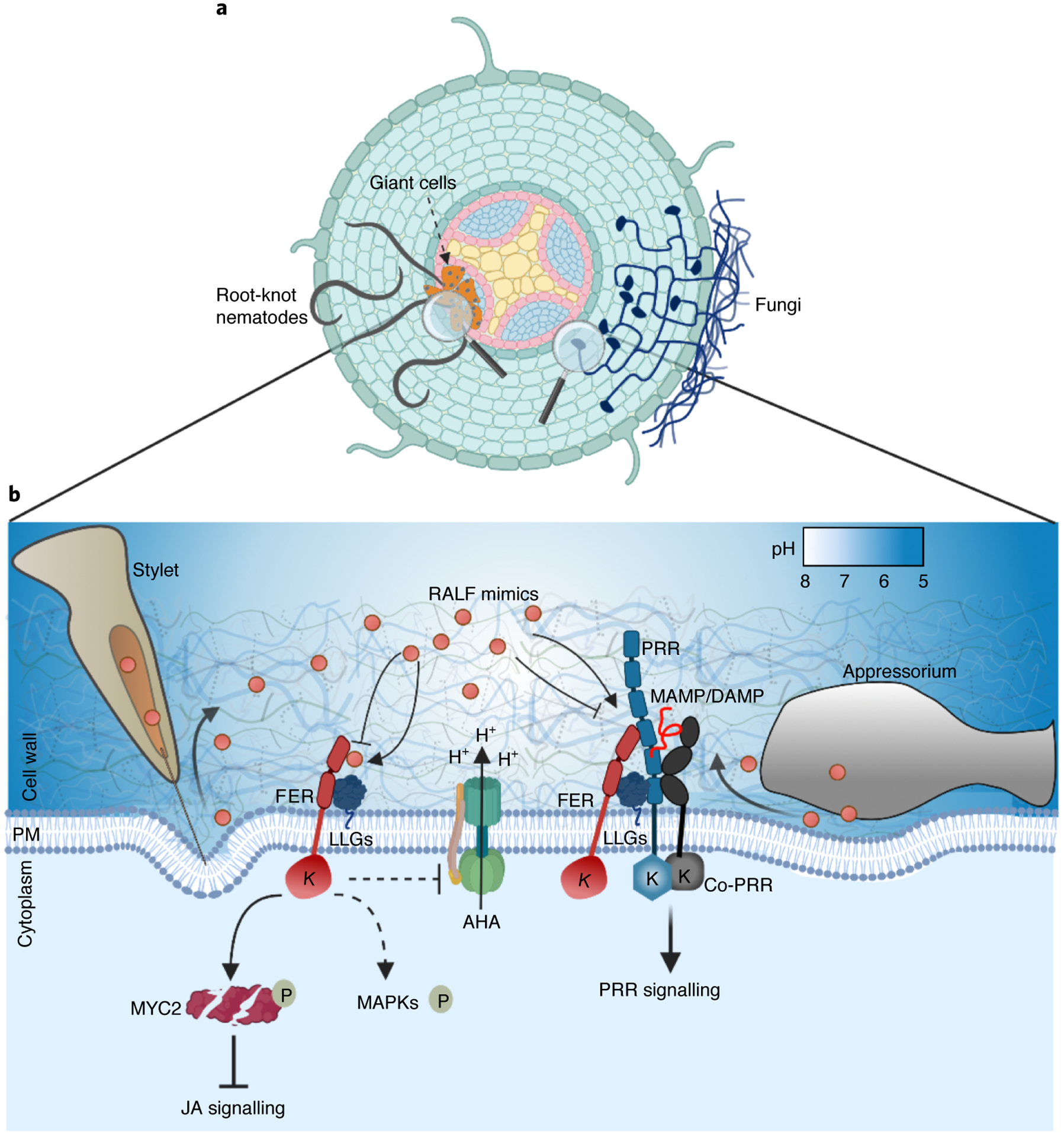
a, Infection diagram of root-knot nematodes and fungi in an Arabidopsis root. Root-knot nematodes induce the redifferentiation of root cells into multinucleated giant cells essential for nematode feeding. b, Secreted RALF mimics (red circles) interact with FER to interfere with PTI responses by modulating the PRR complex assembly, regulate JA signalling by modulating MYC2 stability and induce extracellular alkalinization probably by inhibiting PM H+-ATPases (AHA). RALF mimics are probably secreted through the stylet of nematodes and the appressorium of fungi. MAPK activation triggered by RALF mimics might also be FER mediated. This figure was created with BioRender.
The reported findings point out an evolutionarily conserved role of the RALF–FER module in different host–pathogen interactions. The RALF mimics secreted by pathogens facilitate infections by promoting pathogenicity and suppressing host immunity (Fig. 4). Extracellular alkalinization, the modulation of cell expansion and cell wall modifications could be induced by the secreted RALF mimics, creating a suitable niche to establish invasive structures such as fungal haustoria or nematode-induced giant cells as feeding sites (Fig. 4)90,93. Additionally, immune responses (including the immune sensory complex assembly, ROS production, MAPK activation and hormone signalling) are modulated by RALF mimics to increase the pathogen infection potential. Finally, the growth–defence trade-off could also be regulated by pathogens through FER, promoting plant survival while ensuring a sustained supply of nutrients.
Phylogenetic and coalescent analyses indicate that RALF mimics from fungi and nematodes are intermixed with plant RALFs without an apparent evolutionary origin90,91,93. Most RALF-like sequences identified in pathogens are close to Arabidopsis RALF1. RALF27-like sequences were also found in the poplar pathogen Sphaerulina musiva and Septoria populicola genomes. Interestingly, the closest relative of these two RALF27-like sequences is a poplar RALF27-like gene91. These observations suggest that pathogens might have acquired RALF genes by horizontal gene transfer from their hosts, rather than convergent evolution of RALFs among different organisms. As plant RALFs have been shown to bind different MLRs and regulate diverse plant physiological processes, it would be interesting to investigate whether RALF mimics deployed by pathogens impede these processes with a similar targeting mechanism.
As both pathogenic fungi and nematodes use RALF mimics to hijack FER signalling, plants may have evolved a mechanism to guard FER. A recent study shows that the depletion of FER leads to the recruitment of beneficial Pseudomonas in the complex rhizosphere microbiome86. Notably, the fer mutant changes the soil microbiome structure that promotes plant growth of the next generation. Interestingly, the function of FER in restricting Pseudomonas is independent of its immune scaffold function and JA-mediated immunity but depends on its regulation of ROP–NADPH oxidase-mediated basal ROS production86. The fer mutant in recruiting beneficial microbiota echoes the treatment of RALF23, which negatively regulates FER functions34,47,86. These observations, plus the fact that Arabidopsis RALF23 exhibits high sequence similarity with MiRALF1 and MiRALF3 (ref.93), suggest that invading organisms use RALF mimics to manipulate MLR–ROP–NADPH oxidase signalling pathways to modulate ROS production and thus influence plant immune responses and rhizosphere microbiome structure, which is essential for plant health.
Solving the puzzle—what comes next?
What is the binding and functional specificity of RALF ligands to MLRs?
In recent years, the interactions between the ECDs of MLRs and RALFs have been demonstrated for FER–RALF1/RALF23, ANX1/ANX2–RALF4/RALF19/RALF34, BUPS1/BUPS2–RALF4/RALF19/RALF34 and THE1–RALF34 (refs.29,34,47,94,95). Notably, although THE1 and RALF34 interact, THE1 and RALF1 do not interact under the same conditions, suggesting MLR–RALF binding specificity95. However, cognate ligands for most MLRs are still unknown, and the binding and functional specificity of RALFs remains enigmatic. The binding between MLRs and their RALF ligands seems not to be one-to-one pairwise, as different RALFs can bind to the same receptor, and conversely, one RALF might bind to multiple MLRs. These observations imply that the shared structural features of different RALFs could be recognized by corresponding MLRs, which might have functionally interchangeable ECDs. It would be interesting to perform domain swap analysis among MLRs that recognize the same RALFs, since ECDs of FER, ANX1 and HERK1 that do not have shared ligands are not interchangeable96. Efforts to elucidate the endogenous multimeric protein complex assembly and quantification of the native binding affinity are necessary to fully determine the mechanistic principle of binding specificity between RALFs and MLRs.
RALFs can either activate or repress MLR functions29,34. For instance, RALF23/RALF33 and RALF17 negatively and positively regulate PRR-mediated immunity in an FER-dependent manner, respectively (Fig. 2a)34. JA signalling is upregulated by RALF23 but downregulated by RALF1 and nematode RALF mimics through regulation of MYC2 stability in an FER-dependent manner49,93. How RALFs trigger opposite immune responses through the same MLR remains a mystery. A plausible hypothesis is that RALFs may act as either agonists or antagonists, being the response determined by competition among them to bind the MLRs. Their interplay could cause synergistic or antagonist effects in PRR- and NLR-mediated immunity when perceived by the same MLR receptor. This interplay may not be exclusively limited among RALFs. Recently, it was found that upon pollination, the POLLEN COAT PROTEIN B-class peptides, a class of cysteine-rich peptides, compete with RALF23/RALF33 for binding to the FER–ANJ complex in the stigma papillae, leading to the reduction of stigmatic ROS that allows pollen hydration and germination51. Additionally, competition between Stomagen/EPIDERMAL PATTERNING FACTOR (EPF)-LIKE 9 and EPF2 peptides for binding to LRR–RK ERECTA and its coreceptor LRR–receptor-like protein TOO MANY MOUTHS fine-tunes stomatal patterning97. There is thus an emerging theme of antagonistic regulation of ligand–RK signalling.
How do additional players govern RALF–MLR complex assembly?
Recent findings indicate that the perception of RALF peptides leads to recruitment of additional proteins and formation of a multi-protein heteromeric MLR-containing complex. For instance, RALF peptides induce the association of MLR ECDs with the LLG family glycosylphosphatidylinositol-anchored proteins43–45,47. RALF23 directly interacts with the ECD of FER and LLGs, but no direct interaction between FER and LLGs has been observed, suggesting that RALF23 acts as a molecular glue of the FER–LLG complex47. The N-terminal helix of RALF23 (RALF23N) with the conserved Tyr-Ile-Ser-Tyr motif is sufficient to induce LLG–FER complex formation. Interestingly, RALF23N does not directly interact with FER. Instead, FER interacts with the C-terminus of RALF23, which strengthens the FER–LLG complex47. RALF23 is thus directly recognized by LLGs, resulting in the assembly of the heteromeric complex with FER. This study also suggests that RALF peptides with the conserved Tyr-Ile-Ser-Tyr motif might be recognized analogously by similar or distinct MLRs and LLGs47. A unique heteromeric complex with specific members of RALFs, LLGs and MLRs therefore probably contributes to different downstream signalling events. This notion is supported by the observations that RALF4 promotes interactions between BUPSs/ANXs and LLG2/LLG3 in pollen tube growth, and that a mutation in the Tyr-Ile-Ser-Tyr motif abolishes RALF4 interactions with BUPSs/ANXs and LLGs43,44,98. It remains to be determined whether the LLG1–LET1/LET2 ternary complex controlling plant autoimmunity46 is also regulated by any specific RALFs.
LLGs have been initially proposed to act as coreceptors for MLRs perceiving RALFs43–45. However, their prominent role in direct binding to RALFs in the absence of MLRs challenges this concept47. LLGs are a small group of proteins that redundantly interact with different RALFs43–45. It is possible that they function as the bona fide RALF receptors, whereas MLRs act as coreceptors that contribute to the signalling specificity. Future biochemical, structural and genetic studies are needed to consolidate a conceptual model of the RALF–LLG–MLR functions.
Moreover, members of the cell-wall-associated LRX proteins have been found to interact with RALFs and FER98–102. LRXs possess an EXTENSIN domain with an expected role in crosslinking to cell wall components and an LRR domain that interacts with RALFs and the ECD of FER98–102. Pollen-expressed LRXs (LRX8, 9, 10 and 11) interact with RALF4 and RALF19 and regulate pollen tube integrity upstream of MLR ANX1 and ANX2 (ref.99). The LRR domain of LRXs is structurally close to the ECD of LRR–RKs, such as FLS2 (ref.98). Similar to LLGs, LRXs can directly bind to RALFs with high affinities in the absence of MLRs. Interestingly, the Tyr-Ile-Ser-Tyr motif in RALF4 that is important for its binding to LLGs is not required for its binding to LRX8 or its function in inhibiting pollen tube growth98. It thus seems that LLGs and LRXs sense RALF peptides with mechanistically distinct and mutually exclusive peptide-binding modes that are influenced by pH98. The ability to be differentially recognized by two different receptor modules allows RALF4 and RALF19 to regulate pollen tube growth by participating in two parallel pathways, with one through the LLG–MLR module in activating intracellular signalling and the other through LRXs in sensing extracellular CWI43,98,99. However, how LRX proteins as extracellular proteins induce signalling independent (or at least partially independent) of MLRs remains a mystery.
The triple mutant of LRX3, LRX4 and LRX5, the most highly expressed LRXs in vegetative tissues, exhibits growth and salt-hypersensitive phenotypes reminiscent of fer-4 (refs.100–102), indicating that LRXs are involved in the broad functions of FER. More recently, LRX3, LRX4 and LRX5 were found to be genetically required for RALF17- and RALF23-triggered FER-mediated PTI responses35. Vegetative-expressed LRX1, LRX3, LRX4 and LRX5 were reported to bind RALFs100–102, and ANX1/ANX2 pollen tube signalling components also affect processes influenced by vegetative-expressed LRX1/LRX2 (ref.102). These observations indicate that at least partially similar modes of action with pollen-expressed LRXs in terms of RALF binding and coordination with LLG–MLR-triggered intracellular signalling may exist. Although direct interaction has not been established, the vegetative-expressed LRXs (LRX1, 2, 3, 4 and 5) complex with the ECD of FER, supporting the functional similarity of LRXs and FER102. Notably, the PM association of LRX4 via its LRR domain does not depend on FER102. This could be due to the contribution of additional MLRs to the PM association of LRXs. Alternatively, similar to the scenario with pollen-expressed LRXs98, vegetative-expressed LRXs might not directly interact with MLRs.
How is the formation of specific MLR receptor complexes regulated?
The existence of distinct MLR receptor complexes prompts the question of how the specific complex assembly is regulated. In a biological context, spatio-temporal regulation of the expression of RALFs, MLRs, LLGs, LRXs and other signalling components throughout the plant life cycle and in response to different environmental cues probably occurs, thus generating unique expression patterns that determine the formation of the specific complex at the tissue and cellular levels. For instance, although LLG1/LLG2/LLG3 can complex with the ECD of FER upon RALF23 perception, only the LLG1 expression pattern overlaps considerably with those of FER and RALF23 (refs.34,45,47). The llg1 mutants show similar defects in immunity and growth to those of fer34,45,47,48. The defects of llg2 and llg3 mutants in plant immunity have not been reported. Besides, only llg1, but not llg2 or llg3, regulates SUMM2-mediated autoimmunity46. Conversely, the expression pattern of LLG2/LLG3 but not LLG1 overlaps with those of RALF4/RALF19, BUPS1/BUPS2 and ANX1/ANX2 in pollen29,43,47,103, consistent with their role in pollen tube growth. However, given that ANX1 and ANX2 are important for PTI in leaves36, it cannot be ruled out that LLG2/LLG3 may have a role in plant immunity as a complex partner of ANX1/ANX2.
The composition and assembly of MLR receptor complexes may also be modulated through the compartmentalization of receptors and their partners into specialized nanometre-scale PM platforms designated as nanodomains. PM nanodomains are essential for integrating developmental and immune signals, providing scaffolds for protein–protein interactions104,105. In line with this, it was recently shown that FER is organized into nanodomains on the PM of cotyledon epidermal cells, and together with its cognate ligand RALF23, FER controls mobility and nanodomain organization of FLS2 in modulating PTI signalling35. FER and ANX1/ANX2 (and potentially other MLRs) may therefore be clustered into PM nanodomains together with PRR receptors, coreceptors and other signalling components that complex dynamically in response to different stimuli, assuring proper immune responses. Specific clustering of receptor complexes could also be modulated by a membraneless nano-environment through liquid–liquid phase separation (LLPS), which separates homogenous fluid into a liquid droplet-like structure with rapid, reversible condensation of specific proteins105–107. Active internalization and cleavage of RKs35,108 may facilitate LLPS-mediated compartmentalization of receptor and signalling complexes. Additionally, the regulation of MLR receptor complex protein homeostasis may serve as an alternative mechanism for modulating signalling activation. For instance, FER abundance at the PM is negatively affected by RALF23 and RALF17, which induce FER internalization through endocytosis35. However, since RALF23 and RALF17 oppositely regulate PTI34 and yet both induce FER endocytosis35, the role of RALF-induced FER endocytosis in PRR-mediated immunity is still unclear.
MLRs might also modulate PRR transcripts. RALF1–FER interaction triggers a rapid and massive RNA alternative splicing response by interacting with and phosphorylating GLYCINE-RICH RNA-BINDING PROTEIN7 (GRP7)109. FER-mediated GRP7 phosphorylation promotes GRP7 nuclear accumulation and enhances its mRNA binding and alternative splicing site selection in regulating plant stress responses and growth109. Notably, GRP7 directly associates with the transcripts of PRR FLS2 and EFR and regulates their protein levels110. It remains to be determined whether RALF1–FER–GRP7-regulated alternative splicing is involved in FER-mediated immunity.
Do MLRs function in plant immunity through sensing CWI?
The plant cell wall is the front line of defence to limit pathogens from invading plant cells. To launch a successful infection, pathogens need to breach the physical barriers and alter CWI. The ability to sense cell wall perturbations caused by invading organisms may therefore allow plants to activate prompt defence responses. Mounting evidence indicates that plant immunity and CWI maintenance are intimately linked111. Several MLRs act as sensors of CWI (Fig. 3)21,31,112. For instance, mechanical distortion of the cell wall triggers CWI responses through MLR THE1 (refs.88,112,113), which is required for plant defence against the necrotrophic fungal pathogen B. cinerea87. The disturbance of CWI by isoxaben, a herbicide blocking cellulose production, induces the expression of PROPEPs112, which encode the precursor proteins of the endogenous elicitor peptides (Peps) perceived by PEP1 RECEPTOR 1 (PEPR1) and PEPR2 in triggering PTI responses114. Interestingly, exogenous application of Pep1/Pep3 suppresses accumulation of isoxaben-induced THE1-mediated defence hormones JA and SA, indicating a Pep-dependent negative feedback loop112. In the absence of PEPR1/PEPR2 or other PTI signalling components, CWI-disturbance-induced accumulation of JA and SA is enhanced, suggesting that CWI maintenance may compensate for the immunodeficiency caused by defective PTI signalling112. RALF34 has been reported as a ligand of THE1 in regulating lateral root initiation95. It is possible that the RALF34–THE1 ligand–receptor module might also regulate the immune responses induced by CWI disturbance.
Interestingly, the RALF34–THE1 interaction is pH dependent with an increased binding affinity at an elevated pH, suggesting that extracellular alkalinization is a prerequisite for ligand–receptor interaction95. This observation is consistent with RALF4–LLG binding98. RALF34–THE1 signalling is partially dependent on FER95, which could be related to the capacity of FER to induce extracellular alkalinization upon RALF1 perception, therefore favouring RALF34–THE1 interaction. One can thus hypothesize that through extracellular pH alteration, RALF1–FER might modulate the interaction of THE1 and other MLRs with their cognate ligands, thereby affecting CWI95,111.
Nevertheless, the function of FER in linking plant immunity and CWI may go further. FER has been shown to modulate intracellular vacuolar expansion by sensing the extracellular matrix features through interaction with extracellular LRX proteins101. LRXs interact with FER through their LRR domain and probably bind cell wall components through their EXTENSIN domain, thereby sensing and conveying extracellular signals to the cells101. Similarly, FER-mediated crosstalk between immunity and CWI sensing might be mediated through interaction with LRXs, which are required for FER-regulated PTI signalling35.
The ECDs of FER, BUPS1 and ANX1/ANX2 have been reported to interact with pectin, a critical component of the plant cell wall, suggesting a potential role of MLRs in directly sensing cell wall perturbations115. FER also regulates acclimation to salt stress, which might be associated with its ability to interact with pectin, since the severity of fer’s response to salinity is diminished by treatment with pectin-gelling agents115. Moreover, FER modulates sexual reproduction by regulating ovular pectin levels and ovular pectin-induced nitric oxide production upon pollen tube arrival37. Thus, considering that pectin and pectin-derived products can be derived from cell wall breakdown under pathogen attacks, pectin sensing by FER or other MLRs might integrate CWI maintenance with immune signalling. However, it was recently reported that the association of FER with pectin is dispensable for FER scaffold function in MAMP-induced PRR complex assembly and ROS production35. Nevertheless, the crosstalk between plant immunity and CWI sensing may occur at multiple levels. Recently, BUPS1 was proposed to act in the mechanoperception of acute mechanical stress during pollen tube emergence and is required for mechanical activation of ROP1 GTPase to maintain CWI31. This function is orchestrated with its ligands, RALF4 and RALF19, which amplify the mechanical signal31. A similar module analogous to the RALF–BUPS1–ROP signalling pathway might sense or transduce mechanical forces under the invasive processes of pathogens such as fungal hyphae invasion. Future work needs to further assess how CWI disturbance during infection is connected with plant immune responses and the involvement of specific MLRs in this process.
Do MLRs modulate the growth–defence trade-off?
Plants dynamically re-allocate their resources towards growth or defence upon infection. The activation of defence responses frequently comes with plant growth restriction, hence defining a trade-off116. Plant hormones, including BRs, have emerged as major regulators of the growth–defence trade-off116,117. As mentioned above, connections between MLRs and BR signalling have been observed. HERK1, THE1 and FER, the transcripts of which are upregulated by BR, function cooperatively with the BR pathway to promote cell elongation during vegetative growth81. The role of FER in BR-regulated plant growth is further supported by FER-dependent BR function in controlling hypocotyl elongation of etiolated seedlings82 and by the close correlation between FER-regulated and BR-regulated genes49. BRs are recognized by PM-localized LRR–RK BRI1 receptor, which shares structural similarity with LRR–RK PRRs and requires BAK1 as a coreceptor118. As FER and ANX1/ANX2 interact with and regulate the assembly of PRR–BAK1 complexes (Fig. 2a,b), it remains possible that some MLRs also modulate BR-induced BRI1–BAK1 complex formation or PM nanodomain organization. Furthermore, FER and the MLR ERULUS also mediate auxin signalling, which plays a critical role in plant growth and defence119,120. In addition to a canonical mechanism of auxin signalling via ubiquitin-mediated degradation of transcriptional repressors, growing evidence indicates alternative auxin signalling mechanisms that may involve PM-localized receptors121. It is worth investigating whether MLRs are mechanistically involved in non-canonical auxin signalling pathways.
Apparently, MLRs might connect plant immune and hormone signalling and regulate the growth–defence trade-off in plants (Fig. 3). As potential sensors of CWI perturbation during infection, MLRs could modulate growth or defence responses on the basis of the severity of infection and plant physiological status. Besides, the functions of MLRs in regulating growth and immunity might be uncoupled on the basis of the requirements of their kinase activity. FER kinase activity is indispensable for its functions in controlling root growth and aerial development, JA signalling and vacuolar expansion49,94,101,122,123, but dispensable for ovule fertilization and PTI signalling35,122. These observations point out the existence of diverse mechanisms that MLRs might employ to modulate discrete plant physiological processes. The MLR kinase-independent responses may be associated with a scaffolding role, regulating the assembly and activation of the preformed complexes at the PM, as observed in FER-mediated ligand-induced PRR complex formation34,35. How the MLR kinase-dependent and kinase-independent pathways are differentially activated and coordinated in different biological contexts remains an interesting question to address.
How can we address MLRs as a nexus of PRR- and NLR-mediated immunity?
Although ample evidence has established the essential roles of MLRs in plant immunity, we are far from fully unravelling how MLRs integrate and interconnect plant PTI and ETI. The implementation of innovative approaches with improved spatio-temporal and quantitative separation will help fill in some knowledge gaps. For instance, the association network of MLRs with PRRs and NLRs in the context of tissue expression specificity could be investigated through a sensitized high-throughput interaction assay124. These could provide a blueprint to understand how MLRs connect specific PRRs and NLRs. Additionally, PM compartmentalization into nanodomains is vital for providing scaffolds for receptor complex assembly and signalling specificity insulation104,105. It would be interesting to examine whether and how MLRs play a role in PRR nanodomain formation through super-resolution microscopy. It remains unknown whether NLRs can also be assembled into nanodomains in association with MLRs. Furthermore, despite its infancy, LLPS has been recently suggested as a mechanism to regulate the clustering and signalling of receptor complexes at the PM of plant cells105–107. An exciting interrogation is whether nanodomains and LLPS can cooperatively create nano-environments to modulate the interaction of MLRs with PRRs, NLRs and other signalling partners on the PM, as well as in the extracellular matrix and intracellular spaces.
Recent findings have indicated that coordinated spatio-temporal networks regulate plant immune responses in different cell types125. It is fascinating to evaluate the roles of MLRs along with RALFs, LLGs and LRXs in those regulatory networks in distinct cell types with different extracellular matrices. High-throughput single-cell RNA sequencing and proteomics coupled with high-resolution spatio-temporal single-cell imaging will probably reveal a novel insight into the functional dynamics of MLRs, PRRs and NLRs during PTI and ETI activation. Annotated as protein kinases, the functional specificity of MLRs is probably tailored to post-translational modifications, such as protein phosphorylation and ubiquitylation, which have been shown to regulate PRR complex homeostasis, activation and subcellular dynamics126,127. Quantitative phosphoproteomics and ubiquitylome analyses will probably reveal the phospho- and ubiquitin-codes that regulate the functions of MLRs and their dynamic interactions with PRRs and NLRs.
Concluding remarks
Remarkable progress has been made during recent years in elucidating the versatile functions of MLRs in plant immunity. MLRs are emerging as the essential module that connects plant PTI and ETI by the modulation of PRRs and NLRs at the PM. Recent breakthroughs indicate the mutual potentiation of PTI and ETI11,12; however, how these pathways are mechanistically connected is not well understood. It remains possible that the RALF–LRX–LLG–MLR module may serve as a hub linking two-tiered plant immune receptor-mediated responses on the PM in the context of CWI sensing and coordination of the growth–defence trade-off.
Although the cognate ligands for most MLRs remain to be identified, RALFs seem to be the candidates that modulate the activity of MLRs in plant immunity. Interestingly, RALF homologues have been found in diverse plant-invading organisms, which deploy RALF-like peptides to modulate plant MLR-mediated immune responses in their favour (Fig. 4), highlighting an instrumental role of MLR-mediated signalling in the tug-of-war between hosts and invaders. In addition to RALFs, the functions of MLRs in plant immunity involve the coordinated action of different receptors/coreceptors and signalling components, including LLGs and LRXs, probably through the formation of multimeric protein complexes. The formation of MLR-mediated receptor complexes is probably spatio-temporally fine-tuned by different mechanisms, such as coordinated gene regulatory networks and functional compartmentalization at the PM. However, what we know about the roles of MLRs in plant immunity is just the tip of the iceberg. Deciphering the whole landscape of how MLRs regulate plant immunity will undoubtedly be a burgeoning area of investigation in the coming years.
Acknowledgements
We apologize to those whose work is not cited due to space limitations. This research was supported by grants from the National Institutes of Health (NIH) (no. R01GM092893) to P.H., the NIH (no. R01GM097247) to L.S. and the PEW Latin American Fellows Program to F.A.O.-M.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Jones JD & Dangl JL The plant immune system. Nature 444, 323–329 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Zhou JM & Zhang Y Plant immunity: danger perception and signaling. Cell 181, 978–989 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Couto D & Zipfel C Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol 16, 537–552 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Feng BM, He P & Shan LB From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol 55, 109–137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gust AA, Pruitt R & Nurnberger T Sensing danger: key to activating plant immunity. Trends Plant Sci. 22, 779–791 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Dou D & Zhou JM Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe 12, 484–495 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Cui HT, Tsuda K & Parker JE Effector-triggered immunity: from pathogen perception to robust defense. Annu. Rev. Plant Biol 66, 487–511 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Lolle S, Stevens D & Coaker G Plant NLR-triggered immunity: from receptor activation to downstream signaling. Curr. Opin. Immunol 62, 99–105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuda K & Katagiri F Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol 13, 459–465 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Thomma BPHJ, Nurnberger T & Joosten MHAJ Of PAMPs and effectors: the blurred PTI–ETI dichotomy. Plant Cell 23, 4–15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngou BPM, Ahn HK, Ding P & Jones JDG Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Yuan M et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Y, van Wersch R & Zhang Y Convergent and divergent signaling in PAMP-triggered immunity and effector-triggered immunity. Mol. Plant Microbe Interact 31, 403–409 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Dievart A, Gottin C, Perin C, Ranwez V & Chantret N Origin and diversity of plant receptor-like kinases. Annu. Rev. Plant Biol 71, 131–156 (2020). [DOI] [PubMed] [Google Scholar]
- 15.de Azevedo Manhães AME, Ortiz-Morea FA, He P & Shan L Plant plasma membrane-resident receptors: surveillance for infections and coordination for growth and development. J. Integr. Plant Biol 63, 79–101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westermann J et al. An evolutionarily conserved receptor-like kinases signaling module controls cell wall integrity during tip growth. Curr. Biol 29, 4153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze-Muth P, Irmler S, Schroder G & Schroder J Novel type of receptor-like protein kinase from a higher plant (Catharanthus roseus): cDNA, gene, intramolecular autophosphorylation, and identification of a threonine important for auto- and substrate phosphorylation. J. Biol. Chem 271, 26684–26689 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Nissen KS, Willats WG & Malinovsky FG Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci. 21, 516–527 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Li C, Wu HM & Cheung AY FERONIA and her pals: functions and mechanisms. Plant Physiol. 171, 2379–2392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindner H, Muller LM, Boisson-Dernier A & Grossniklaus U CrRLK1L receptor-like kinases: not just another brick in the wall. Curr. Opin. Plant Biol 15, 659–669 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Franck CM, Westermann J & Boisson-Dernier A Plant malectin-like receptor kinases: from cell wall integrity to immunity and beyond. Annu. Rev. Plant Biol 69, 301–328 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Yang H et al. Malectin/malectin-like domain-containing proteins: a repertoire of cell surface molecules with broad functional potential. Cell Surf. 7, 100056 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escobar-Restrepo JM et al. The FERONIA receptor-like kinase mediates male–female interactions during pollen tube reception. Science 317, 656–660 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Duan Q, Kita D, Li C, Cheung AY & Wu HM FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl Acad. Sci. USA 107, 17821–17826 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J et al. FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proc. Natl Acad. Sci. USA 113, E5519–E5527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boisson-Dernier A et al. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136, 3279–3288 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki S et al. ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol 19, 1327–1331 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Boisson-Dernier A et al. ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 11, e1001719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge Z et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358, 1596–1600 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu L et al. The Arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil. Plant J. 95, 474–486 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Zhou X et al. Membrane receptor-mediated mechano-transduction maintains cell integrity during pollen tube growth within the pistil. Dev. Cell 56, 1030–1042 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Blackburn MR, Haruta M & Moura DS Twenty years of progress in physiological and biochemical investigation of RALF peptides. Plant Physiol. 182, 1657–1666 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler SA et al. Conserved molecular components for pollen tube reception and fungal invasion. Science 330, 968–971 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Stegmann M et al. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355, 287–289 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Gronnier J et al. Regulation of immune receptor kinases plasma membrane nanoscale landscape by a plant peptide hormone and its receptors. Preprint at bioRxiv 10.1101/2020.07.20.212233 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mang H et al. Differential regulation of two-tiered plant immunity and sexual reproduction by ANXUR receptor-like kinases. Plant Cell 29, 3140–3156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan QH et al. FERONIA controls pectin- and nitric oxide-mediated male–female interaction. Nature 579, 561–566 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Ngo QA, Vogler H, Lituiev DS, Nestorova A & Grossniklaus U A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell 29, 491–500 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Zou Y et al. Transcriptional regulation of the immune receptor FLS2 controls the ontogeny of plant innate immunity. Plant Cell 30, 2779–2794 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DM et al. A malectin-like receptor kinase regulates cell death and pattern-triggered immunity in soybean. EMBO Rep. 21, e50442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z et al. Mutations of two FERONIA-like receptor genes enhance rice blast resistance without growth penalty. J. Exp. Bot 71, 2112–2126 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang YY et al. Identification of FERONIA-like receptor genes involved in rice–Magnaporthe oryzae interaction. Phytopathology 2, 1–10 (2020). [Google Scholar]
- 43.Feng HQ et al. LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 regulate pollen tube growth as chaperones and coreceptors for ANXUR/BUPS receptor kinases in Arabidopsis. Mol. Plant 12, 1612–1623 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Ge Z et al. LLG2/3 are co-receptors in BUPS/ANX-RALF signaling to regulate Arabidopsis pollen tube integrity. Curr. Biol 29, 3526–3265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4, e06587 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y et al. A trimeric CrRLK1L–LLG1 complex genetically modulates SUMM2-mediated autoimmunity. Nat. Commun 11, 4859 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao Y et al. Mechanisms of RALF peptide perception by a heterotypic receptor complex. Nature 572, 270–274 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Shen Q, Bourdais G, Pan H, Robatzek S & Tang D Glycosylphosphatidylinositol-anchored protein LLG1 associates with and modulates FLS2 to regulate innate immunity. Proc. Natl Acad. Sci. USA 114, 5749–5754 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo H et al. FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Curr. Biol 28, 3316–3324 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Liang X & Zhou JM Receptor-like cytoplasmic kinases: central players in plant receptor kinase-mediated signaling. Annu. Rev. Plant Biol 69, 267–299 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Liu C et al. Pollen PCP-B peptides unlock a stigma peptide–receptor kinase gating mechanism for pollination. Science 372, 171–175 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Cheng YT et al. Stability of plant immune-receptor resistance proteins is controlled by SKP1-CULLIN1-F-box (SCF)-mediated protein degradation. Proc. Natl Acad. Sci. USA 108, 14694–14699 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gou M et al. The F-box protein CPR1/CPR30 negatively regulates R protein SNC1 accumulation. Plant J. 69, 411–420 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Wu Z et al. Plant E3 ligases SNIPER1 and SNIPER2 broadly regulate the homeostasis of sensor NLR immune receptors. EMBO J. 39, e104915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung EH et al. Specific threonine phosphorylation of a host target by two unrelated type III effectors activates a host innate immune receptor in plants. Cell Host Microbe 9, 125–136 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Elmore JM, Lin ZJD & Coaker G A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe 9, 137–146 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung EH, El-Kasmi F, He YJ, Loehr A & Dangl JL A plant phosphoswitch platform repeatedly targeted by type III effector proteins regulates the output of both tiers of plant immune receptors. Cell Host Microbe 16, 484–494 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Du CQ et al. Receptor kinase complex transmits RALF peptide signal to inhibit root growth in Arabidopsis. Proc. Natl Acad. Sci. USA 113, E8326–E8334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J et al. The malectin-like receptor-like kinase LETUM1 modulates NLR protein SUMM2 activation via MEKK2 scaffolding. Nat. Plants 6, 1106–1115 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y et al. Receptor-like kinases MDS1 and MDS2 promote SUMM2-mediated immunity. J. Integr. Plant Biol 63, 277–282 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Bi G & Zhou JM MAP kinase signaling pathways: a hub of plant–microbe interactions. Cell Host Microbe 21, 270–273 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Devendrakumar KT, Li X & Zhang YL MAP kinase signalling: interplays between plant PAMP- and effector-triggered immunity. Cell. Mol. Life Sci 75, 2981–2989 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asai T et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Petersen M et al. Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103, 1111–1120 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Ichimura K, Casais C, Peck SC, Shinozaki K & Shirasu K MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J. Biol. Chem 281, 36969–36976 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Suarez-Rodriguez MC et al. MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol. 143, 661–669 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao M et al. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 18, 1190–1198 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z et al. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 11, 253–263 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Takagi M et al. Disruption of the MAMP-induced MEKK1–MKK1/MKK2–MPK4 pathway activates the TNL immune receptor SMN1/RPS6. Plant Cell Physiol. 60, 778–787 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Yang Y et al. RNAi-based screen reveals concerted functions of MEKK2 and CRCK3 in plant cell death regulation. Plant Physiol. 183, 331–344 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galindo-Trigo S et al. CrRLK1L receptor-like kinases HERK1 and ANJEA are female determinants of pollen tube reception. EMBO Rep. 21, e48466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong Q et al. The MEKK1–MKK1/MKK2–MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell 24, 2225–2236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su SH et al. Deletion of a tandem gene family in Arabidopsis: increased MEKK2 abundance triggers autoimmunity when the MEKK1–MKK1/2–MPK4 signaling cascade is disrupted. Plant Cell 25, 1895–1910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z et al. The NLR protein SUMM2 senses the disruption of an immune signaling MAP kinase cascade via CRCK3. EMBO Rep. 18, 292–302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nitta Y et al. MEKK2 inhibits activation of MAP kinases in Arabidopsis. Plant J. 103, 705–714 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Wang J et al. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364, aav5870 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Wang J et al. Ligand-triggered allosteric ADP release primes a plant NLR complex. Science 364, eaav5868 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Wan WL, Kim ST, Castel B, Charoennit N & Chae E Genetics of autoimmunity in plants: an evolutionary genetics perspective. N. Phytol 3, 1215–1233 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Bürger M & Chory J Stressed out about hormones: how plants orchestrate immunity. Cell Host Microbe 26, 163–172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robert-Seilaniantz A, Grant M & Jones JDG Hormone crosstalk in plant disease and defense: more than just JASMONATE–SALICYLATE antagonism. Annu. Rev. Phytopathol 49, 317–343 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Guo H et al. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 106, 7648–7653 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deslauriers SD & Larsen PB FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant 3, 626–640 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Liao H, Tang R, Zhang X, Luan S & Yu F FERONIA receptor kinase at the crossroads of hormone signaling and stress responses. Plant Cell Physiol. 58, 1143–1150 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Zheng XY et al. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11, 587–596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chico JM et al. CUL3(BPM) E3 ubiquitin ligases regulate MYC2, MYC3, and MYC4 stability and JA responses. Proc. Natl Acad. Sci. USA 117, 6205–6215 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song Y et al. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat. Plants 7, 644–654 (2021). [DOI] [PubMed] [Google Scholar]
- 87.Qu S, Zhang X, Song Y, Lin J & Shan X THESEUS1 positively modulates plant defense responses against Botrytis cinerea through GUANINE EXCHANGE FACTOR4 signaling. J. Integr. Plant Biol 59, 797–804 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Fujikura U et al. Atkinesin-13A modulates cell-wall synthesis and cell expansion in Arabidopsis thaliana via the THESEUS1 pathway. PLoS Genet. 10, e1004627 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ronald P & Joe A Molecular mimicry modulates plant host responses to pathogens. Ann. Bot 121, 17–23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masachis S et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol 1, 16043 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Thynne E et al. Fungal phytopathogens encode functional homologues of plant rapid alkalinization factor (RALF) peptides. Mol. Plant Pathol 18, 811–824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wood AKM, Walker C, Lee WS, Urban M & Hammond-Kosack KE Functional evaluation of a homologue of plant rapid alkalinisation factor (RALF) peptides in Fusarium graminearum. Fungal Biol. 124, 753–765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang X et al. Nematode-encoded RALF peptide mimics facilitate parasitism of plants through the FERONIA receptor kinase. Mol. Plant 13, 1434–1454 (2020). [DOI] [PubMed] [Google Scholar]
- 94.Haruta M, Sabat G, Stecker K, Minkoff BB & Sussman MR A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343, 408–411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gonneau M et al. Receptor kinase THESEUS1 is a Rapid Alkalinization Factor 34 receptor in Arabidopsis. Curr. Biol 28, 2452–2458 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Kessler SA, Lindner H, Jones DS & Grossniklaus U Functional analysis of related Cr RLK 1L receptor‐like kinases in pollen tube reception. EMBO Rep. 16, 107–115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee JS et al. Competitive binding of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moussu S et al. Structural basis for recognition of RALF peptides by LRX proteins during pollen tube growth. Proc. Natl Acad. Sci. USA 117, 7494–7503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mecchia MA et al. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358, 1600–1603 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Zhao C et al. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl Acad. Sci. USA 115, 13123–13128 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dünser K et al. Extracellular matrix sensing by FERONIA and leucine‐rich repeat extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J. 38, e100353 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herger A et al. Overlapping functions and protein–protein interactions of LRR-extensins in Arabidopsis. PLoS Genet. 16, e1008847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsukamoto T, Qin Y, Huang Y, Dunatunga D & Palanivelu R A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 62, 571–588 (2010). [DOI] [PubMed] [Google Scholar]
- 104.Bucherl CA et al. Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife 6, 25114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jaillais Y & Ott T The nanoscale organization of the plasma membrane and its importance in signaling: a proteolipid perspective. Plant Physiol. 182, 1682–1696 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cuevas-Velazquez CL & Dinneny JR Organization out of disorder: liquid–liquid phase separation in plants. Curr. Opin. Plant Biol 45, 68–74 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Emenecker RJ, Holehouse AS & Strader LC Emerging roles for phase separation in plants. Dev. Cell 55, 69–83 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou J et al. Proteolytic processing of SERK3/BAK1 regulates plant immunity, development, and cell death. Plant Physiol. 180, 543–558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang L et al. RALF1–FERONIA complex affects splicing dynamics to modulate stress responses and growth in plants. Sci. Adv 6, eaaz1622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nicaise V et al. Pseudomonas HopU1 modulates plant immune receptor levels by blocking the interaction of their mRNAs with GRP7. EMBO J. 32, 701–712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vaahtera L, Schulz J & Hamann T Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 5, 924–932 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Engelsdorf T et al. The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. Sci. Signal 11, 536 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Hématy K et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol 17, 922–931 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Bartels S et al. The family of Peps and their precursors in Arabidopsis: differential expression and localization but similar induction of pattern-triggered immune responses. J. Exp. Bot 64, 5309–5321 (2013). [DOI] [PubMed] [Google Scholar]
- 115.Feng W et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol 28, 666–675 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ortiz-Morea FA, He P, Shan L & Russinova E It takes two to tango—molecular links between plant immunity and brassinosteroid signalling. J. Cell Sci 133, 246728 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huot B, Yao J, Montgomery BL & He SY Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Mol. Plant 7, 1267–1287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim E-J & Russinova E Brassinosteroid signalling. Curr. Biol 30, R294–R298 (2020). [DOI] [PubMed] [Google Scholar]
- 119.Schoenaers S et al. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol 28, 722–732 (2018). [DOI] [PubMed] [Google Scholar]
- 120.Barbez E, Dunser K, Gaidora A, Lendl T & Busch W Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 114, E4884–E4893 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McLaughlin HM, Ang ACH & Østergaard L Noncanonical auxin signaling. Cold Spring Harb. Perspect. Biol 13, 039917 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haruta M, Gaddameedi V, Burch H, Fernandez D & Sussman MR Comparison of the effects of a kinase‐dead mutation of FERONIA on ovule fertilization and root growth of Arabidopsis. FEBS Lett. 592, 2395–2402 (2018). [DOI] [PubMed] [Google Scholar]
- 123.Chakravorty D, Yu Y & Assmann SM A kinase‐dead version of FERONIA receptor‐like kinase has dose‐dependent impacts on rosette morphology and RALF 1‐mediated stomatal movements. FEBS Lett. 592, 3429–3437 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smakowska-Luzan E et al. An extracellular network of Arabidopsis leucine-rich repeat receptor kinases. Nature 553, 342–346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rich-Griffin C et al. Regulation of cell type-specific immunity networks in Arabidopsis roots. Plant Cell 32, 2742–2762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kadota Y et al. Quantitative phosphoproteomic analysis reveals common regulatory mechanisms between effector- and PAMP-triggered immunity in plants. N. Phytol 221, 2160–2175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma X et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature 581, 199–203 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


