Abstract
Background:
Asthma and obesity are major, interconnected public health challenges that usually have their origins in childhood, and for which the relationship is strengthened among those with insulin resistance.
Objective:
To determine whether high insulin in early life confers increased longitudinal risk for asthma independent of body mass index (BMI).
Methods:
The study used data from the Tucson Children’s Respiratory Study (TCRS) and the Avon Longitudinal Study of Parents and Children (ALSPAC). Non-fasting insulin was measured in TCRS participants at age 6 and fasting insulin in ALSPAC participants at age 8. Physician-diagnosed active asthma was determined at baseline and at subsequent assessments up to age 36 years in TCRS and 17 years in ALSPAC.
Results:
In TCRS, high insulin (upper quartile) at age 6 was associated with increased odds of having active asthma from ages 8 to 36 compared to low insulin (OR 1.98, [1.28–3.05], p=0.002). Similarly, in ALSPAC, high insulin was associated with a significantly higher risk of active asthma from ages 11 to 17 compared to low insulin (1.59 [1.12–2.27], p=0.009). These findings were independent of baseline BMI in both cohorts, and were not related to other demographic and asthma risk factors nor other tested markers of systemic inflammation and metabolic syndrome.
Conclusions:
In two separate birth cohorts, higher blood insulin level in early childhood was associated with increased risk of active asthma through adolescence and adulthood, independent of BMI. High insulin indicates a novel mechanism for asthma development, which may be a target for intervention.
Keywords: Asthma, Insulin, Metabolic syndrome, Obesity
Introduction:
Asthma is a major health challenge and the common label used to diagnose a heterogeneous set of conditions that most often begin in childhood and are associated with a variety of genetic, environmental and developmental risk factors(1). In the last two decades, obesity has been consistently found to be associated with asthma, both in children(2) and adults(3). The exact nature and direction of this association is unknown. Obesity is postulated to increase the risk of asthma by affecting lung mechanics(4), increasing type 2 inflammation(5), increasing type 1 inflammation(6), and inducing resistance to corticosteroid-based treatment(7–9) and beta-agonists(10). However, patients with asthma are also more likely to become obese after diagnosis(11, 12). This risk has been attributed to a combination of factors, including the use of systemic corticosteroids and reduced physical activity levels(13). Weight loss variably benefits obese patients with asthma(14), suggesting that the relation between obesity and asthma may not result directly from increased adipose tissue but may be mediated by biological mechanisms that are common to both asthma and obesity, such as insulin resistance.
Insulin resistance is a metabolic disorder strongly linked with obesity(15), and there is robust evidence that systemic inflammation generated by adipose tissue may trigger insulin resistance(16). Data from the Avon Longitudinal Study of Parents and Children (ALSPAC) have previously shown that greater weight gain in the first three years of life is related to higher body mass index (BMI) and fasting insulin levels at age 8(17). However, genetic studies have also raised the possibility that carbohydrate-generated insulin secretion may play a causal role in obesity(18), suggesting that the association between obesity and high insulin may be bidirectional. Interestingly, a cross-sectional analysis of the National Health and Nutrition Examination Survey revealed that the relation of obesity to asthma prevalence was strongest among persons with insulin resistance(19). These results raise the possibility that insulin resistance may have an effect on asthma risk that is independent of obesity. However, the influence of insulin resistance in early life on development of asthma is not yet known.
We hypothesized that increased insulin levels in early life contribute to the risk for the development of asthma, independent from BMI. To test this hypothesis, we used longitudinal data from two separate cohorts- the Tucson Children’s Respiratory Study (TCRS) and ALSPAC- in which serum insulin levels were measured in childhood and longitudinal follow up for asthma was performed.
Methods:
TCRS cohort:
TCRS is a non-selected birth cohort enrolled from 1980–1984, which was designed to assess early life risk factors for subsequent respiratory outcomes, and which has had continuous follow up for over 36 years(20). The enrollment process and study design have been presented previously(21). Participants of TCRS underwent the first in-depth study visit at age 6 (6.1 years [standard deviation (SD), 0.7 years]). Body mass index (BMI) was assessed at age 6 using standard methods in 303 of the participants included in these analyses; participant-reported height and weight was available for an additional 35 participants. BMI (zBMI) was calculated and z-scored based on CDC growth chart reference tables(22). Blood was collected for serologic studies; participants were not required to be fasting at the time of blood collection. Physician diagnosed active asthma at age 6 and in subsequent surveys through age 36 was defined as a physician diagnosis plus active symptoms in the past year as previously reported(23). Lung function testing was performed at age 6 (V̇maxFRC)(24) through spirometry as previously reported(25–27). V̇maxFRC at age six was adjusted for height and z-scored. At age 6, bronchial hyper-reactivity (BHR) to cold, dry air was defined as a drop of V̇maxFRC greater than 41.1%, the 90th percentile for decline for reference children(28, 29). At age 6, 189 participants underwent BHR testing, which was not performed in children with history of active respiratory disease(30); 152 were not tested and had a higher prevalence of asthma (20.4%) than those tested (4.8%) and were included in the models as a third category for BHR. Allergy skin prick testing was performed at age 6. Atopy was defined as one or more positive skin test(29). Serum IgE was measured as previously reported(31).
Non-fasting insulin, leptin, c-reactive protein (CRP), and interleukin (IL)-6 serum levels were measured by multiplex (Human Multi-Analyte Profile panel version 1.6, Myriad Rules Based Medicine, Austin TX) on all individuals for whom serum collected at the age 6 visit was available for analysis (n=383). Insulin analyses were limited to measurements in a single batch of serum samples measured over 7 plates (n=342). There was no relation between plate and insulin level (p=0.69 by Kruskall-Wallis). Results for insulin from RBM assays were corroborated by ELISA (R&D Systems, Minneapolis, USA) in a randomly selected subset of the same samples used for the RBM assays (see figure e1 in the Online Repository). Insulin levels at age 6 were divided into quartiles, separately by sex. In 98 of the 342 included samples (29%), insulin levels were lower than the minimum detectable concentration (0.67 uIU/ml). IL-6 levels were undetectable in 75% of participants measured, thus divided into detectable and undetectable groups for purposes of analysis.
This research was approved by the Institutional Review Board of the University of Arizona, and informed consent/assent was obtained from/for all subjects.
ALSPAC cohort:
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a birth cohort that recruited 14,541 pregnant woman from Bristol, UK, with expected dates of delivery between April 1991 and December 1992(32–34). A study website contains details of all the data that are available through a fully searchable data dictionary and variable search tool: http://www.bristol.ac.uk/alspac/researchers/our-data/. Additional information on the cohort is available in the Online Repository Methods.
Doctor diagnosed active asthma was assessed by questionnaire at ages 8, 11, 14, 15, and 17(35, 36). Body weight and height were measured using standardized methods(37). Calculated BMI from these measurements at ages 7 and 9 were averaged for each participant to approximate the child’s BMI around age 8. If both measurements were not available, BMI at age 7 or age 9 was included. Z-scored BMI (zBMI) based on the UK growth chart reference tables was used for analysis(22). Spirometry was performed according to American Thoracic Society and European Respiratory Society criteria at age 8(33, 38). Methacholine challenge was assessed at age 8, the dose at which FEV1 dropped by 20% was calculated(39) and methacholine responsiveness was defined(40). From the transformed least squares dose response slope calculated from the percent decline in FEV1 versus cumulative dose, the participants were categorized into <=0 slope and the >0 slope divided into tertiles. Allergy skin prick testing was performed at age 7.5 using a panel of extracts(41). Cohort-specific cutoffs were used, defined as a skin test wherein the sum of the longest diameter plus the diameter of the orthogonal was at least 1mm was considered positive. Atopy was defined as one or more positive skin test.
At age 8 years (mean ± SD age, 8.2 ± 0.1 years; range, 8.0–8.5 years), a randomly selected subsample attended the research clinic in the morning while fasting from at least midnight the previous day. A venous blood sample was collected(37). Methods used for serologic studies are as previously published(17). Insulin was measured by ELISA immunoassay (DSL) with no cross-reactivity with pro-insulin up to 1000 pmol/l. Fasting insulin levels at age 8 were divided into quartiles, separately by sex. Of these, 9.6% (82/857) had undetectable levels of insulin (minimum detectable concentration 0.26 uIU/ml).
Ethical approval for ALSPAC was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Signed consent was obtained from a parent, and verbal assent was obtained from the child.
Statistical methods:
Analyses were performed using Stata (Version 16, StataCorp). Demographic variables were compared using chi-square or ANOVA. Fisher Exact Test was used when appropriate. Longitudinal risk for asthma was determined using generalized estimating equations. For each cohort, we grouped insulin levels into quartiles separately for males and females. The lower three quartiles were combined for males and for females to create a dichotomous insulin variable for each sex, highest quartile vs. lower three quartiles. The sex specific groups were then combined to create a one dichotomous insulin variable: highest quartile, hereafter called ‘high insulin’, and lower three quartiles combined, hereafter called ‘low insulin’. Interaction terms were tested by using continuous z-scores of BMI and testing for interaction with the dichotomous insulin variable. For TCRS, because of the number of time points, the exchangeable structure had the lowest QIC. The lowest QIC for ALSPAC was with unstructured correlation structure. In TCRS, cold air BHR was included in the model as category 0 for no BHR, 1 for BHR positive, and 2 for those who did not do BHR testing. In the model, category 1 was compared to category 0 and category 2 was compared to category 0.
Results
Analyses in TCRS
In the Tucson Children’s Respiratory Study, levels of non-fasting insulin were measured in serum samples collected at the age 6 visit in 342 subjects who had subsequent asthma assessments through age 36. None of these participants were using inhaled or systemic steroids at the time of the age 6 visit (circa 1988). Participants included in this analysis (n=342) were more likely to have non-Hispanic White and Hispanic White ethnicity and less likely to have maternal asthma history than those excluded for lack of insulin measurement or asthma information (n=904) (see table e1 in the Online Repository).
Non-fasting insulin levels were higher in females (n=179) than in males (n= 163) (median (interquartile range) 1.4 (0.67, 2.8)) uIU/mL vs 1.1 (0.33, 2.60) uIU/mL, respectively, p=0.045 by Kruskall-Wallis). As described in the methods, we defined groups with high insulin, defined as the top quartile of insulin for each gender, and low insulin, representing the lower three quartiles. Maternal characteristics and early life exposures were similar among participants with high insulin and low insulin (Table 1).
Table 1.
TCRS: Characteristics of study participants by age 6 high and low insulin groups. Number of participants in each group are noted in the table when they differ from the overall insulin groups.
| Characteristics | Group | Low Insulin (N=254) |
High Insulin (N=88) |
p | ||
|---|---|---|---|---|---|---|
| % | n | % | n | |||
| Sex | Male | 48.0 | 122 | 46.6 | 41 | 0.816 |
| Ethnicity | Non-Hisp White | 61.4 | 156 | 67.0 | 59 | |
| Hisp White | 25.2 | 64 | 21.6 | 19 | ||
| Other | 13.4 | 34 | 11.4 | 10 | 0.642 | |
| Maternal asthma | Yes | 7.2 | 18/250 | 10.3 | 9/87 | 0.352 |
| Maternal smoking during pregnancy | Yes | 14.9 | 37/249 | 15.1 | 13/86 | 0.954 |
| Maternal age at delivery | Mean (95%CI) | 27.4 (26.9,27.9) | 254 | 28.1 (27.2, 29.0) | 88 | 0.198 |
| Ever breastfed in the first 6 months | Yes | 85.3 | 215/252 | 84.9 | 73/86 | 0.922 |
| Day care first 6 months | Yes | 8.1 | 20/248 | 8.2 | 7/85 | 0.960 |
| Indoor dogs in infancy | Yes | 34.0 | 86/253 | 27.3 | 24/88 | 0.245 |
| Maternal smoking first year after birth | Yes | 17.9 | 45/252 | 15.9 | 14/88 | 0.678 |
| Characteristics, age 6 | ||||||
| BMI 1 , CDC z-score (SD) | Mean (95%CI) | 0.11 (−0.04, 0.27) | 251 | 0.45 (0.22, 0.69) | 87 | 0.026 |
| BHR 1 | Yes | 14.2 | 21/148 | 29.3 | 12/41 | 0.024 |
| Asthma | Yes | 11.1 | 28/253 | 13.6 | 12/88 | 0.519 |
| Skin test positivity | Yes | 36.7 | 92/251 | 46.0 | 40/87 | 0.125 |
| Total IgE (IU/mL) | GM (95%CI) | 36.7 (29.7,45.4) | 252 | 45.3 (31.0, 66.1) | 88 | 0.329 |
| V̇maxFRC (ml/s) | GM (95%CI) | 1198 (1149, 1249) | 198 | 1102 (1029, 1180) | 64 | 0.047 |
BMI included as z-scores from CDC reference tables; BHR included as positive and negative response to cold air challenge (30); V̇maxFRC included as z-scores of height adjusted values (24)
Abbreviations: BMI - body mass index; BHR - bronchial hyper-responsiveness; V̇maxFRC - maximal expiratory flow at functional residual capacity; IgE - immunoglobulin E; GM - geometric mean
When compared with children with low insulin, those with high insulin were more likely to have bronchial hyper-responsiveness to cold air (29.3%% [n=12/41] vs. 14.2% [21/148], p=0.024) at age 6 (Table 1). There was no difference in age 6 asthma prevalence (p=0.519), positive skin testing to aeroallergens (p=0.125), or total IgE (p=0.329) between the high and low insulin groups. Compared with the low insulin group, the high insulin group had a lower V̇maxFRC (1102ml/s [1029, 1180] n=64 vs. 1198ml/s [1149, 1249] n=198; p=0.047) and a higher mean BMI (16.6 [16.0, 17.1] vs. 15.8 [15.6, 16.0], p=0.002) at age 6.
High insulin at age 6 was associated with higher prevalence of active asthma at ages 8 to 36 years compared to low insulin (Figure 1). In a longitudinal model, high insulin nearly doubled the odds of having active asthma from ages 8 to 36 compared to low insulin (OR 1.98, [1.28–3.05], p=0.002, n=342; Table 2). The relation between high insulin and active asthma remained significant after adjustment for age 6 zBMI and sex (OR 1.84 [1.18–2.87], p=0.007, n=338), while that between age 6 zBMI and asthma was not significant in this model (OR 1.08 [0.90–1.30], p=0.419). There was no interaction between zBMI and insulin for asthma risk. A model including age 6 zBMI, BHR and z-scored V̇maxFRC did not appreciably change the relation between high insulin and subsequent asthma (OR 2.44 [1.50–3.97], p<0.001, n=262). Serum leptin, C-reactive protein (CRP), and IL-6 at age 6 were not related to asthma risk, and adjustment for these markers had no effect on the insulin-asthma association (see Table e2 in the Online Repository).
Figure 1.
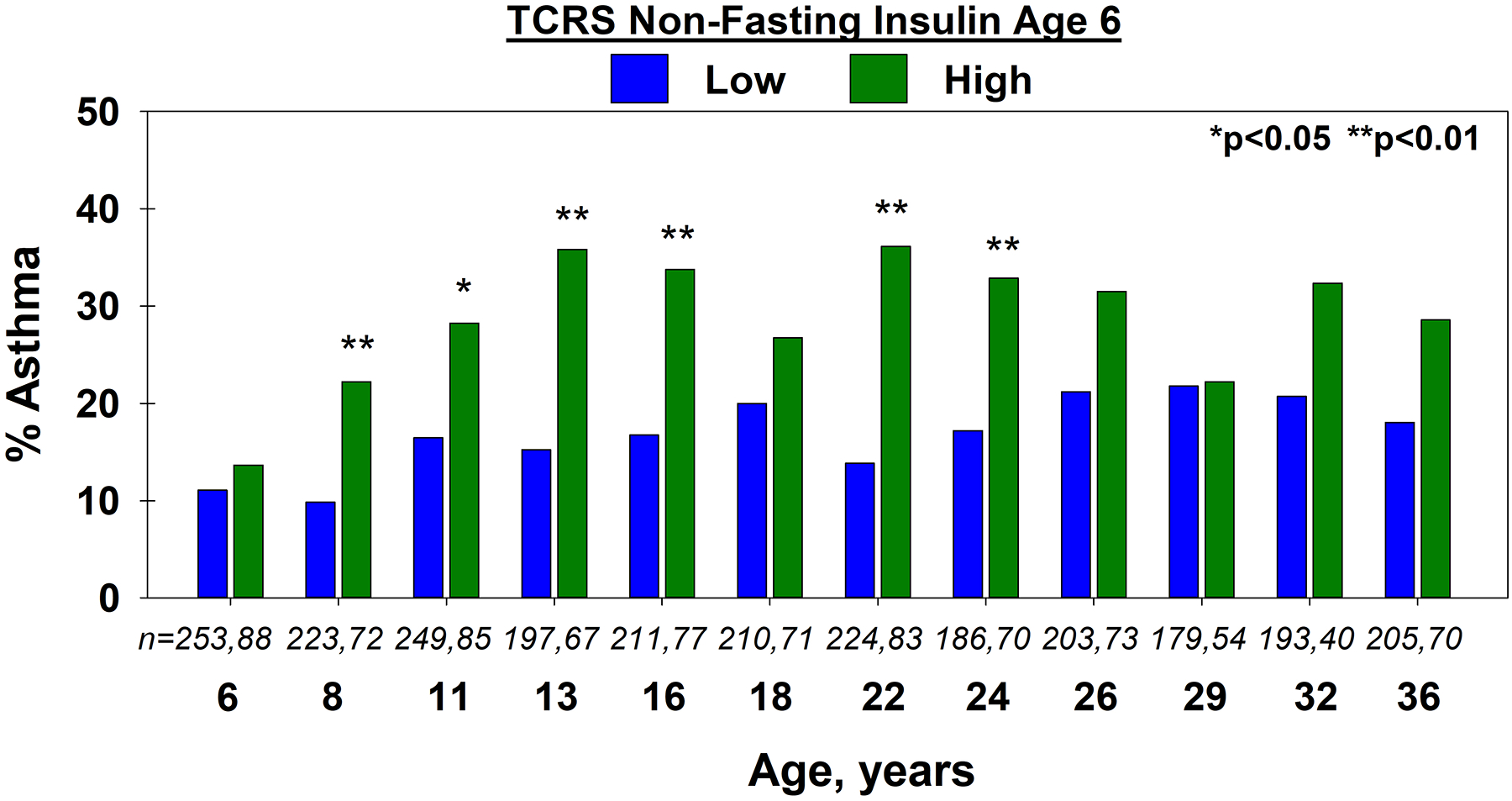
TCRS: Proportion of participants with asthma at each age, by low vs. high non-fasting insulin, in the TCRS cohort. Number of participants in the low and high insulin groups are shown in italics.
Table 2.
TCRS: Odds of subsequent asthma for ages 8–36 years, comparing high non-fasting insulin with low insulin at age 6. All models adjusted for sex; models 2–5 adjusted for z-score BMI1 at age 6. Models 1 and 2 include all participants, models 3 and 4 are limited to those who underwent the specified testing at age 6, model 5 is limited to those with no asthma at age 6.
| Models | Odds Ratio | 95% CI | p | n | Observations | |
|---|---|---|---|---|---|---|
| 1 | All participants | |||||
| High Insulin | 1.98 | 1.28, 3.05 | 0.002 | 342 | 3080 | |
| Female | 0.91 | 0.60, 1.37 | 0.646 | |||
| 2 | All participants with BMI | |||||
| High Insulin | 1.84 | 1.18, 2.87 | 0.007 | 338 | 3046 | |
| Female | 0.94 | 0.62, 1.41 | 0.756 | |||
| zBMI | 1.08 | 0.90, 1.30 | 0.419 | |||
| 3 | Adjusted for age 6 BHR 1 | |||||
| High Insulin | 1.70 | 1.09, 2.64 | 0.018 | 338 | 3046 | |
| BHR Positive | 2.17 | 1.11, 4.26 | 0.024 | |||
| BHR, not tested | 1.86 | 1.19, 2.90 | 0.006 | |||
| Female | 0.98 | 0.65, 1.47 | 0.921 | |||
| zBMI | 1.08 | 0.90, 1.29 | 0.396 | |||
| 4 | Adjusted for age 6 V̇maxFRC 1 | |||||
| High Insulin | 2.44 | 1.50, 3.97 | <0.001 | 262 | 2374 | |
| V̇maxFRC1 (SD) | 0.74 | 0.59, 0.94 | 0.012 | |||
| Female | 0.78 | 0.49, 1.24 | 0.294 | |||
| zBMI | 1.13 | 0.90,1.41 | 0.293 | |||
| 5 | Limited to those with no asthma at age 6 | |||||
| High Insulin | 1.80 | 1.12, 2.92 | 0.016 | 298 | 2678 | |
| Female | 1.25 | 0.79, 1.98 | 0.337 | |||
| zBMI | 1.26 | 1.01, 1.58 | 0.045 | |||
BMI included as z-scores from CDC reference tables; BHR included as positive and negative response to cold air challenge (30); V̇maxFRC included as z-scores of height adjusted values (24)
Abbreviations: BMI - body mass index; BHR - bronchial hyper-responsiveness; V̇maxFRC - maximal expiratory flow at functional residual capacity
To exclude the possibility for baseline asthma diagnosis to influence the outcome of interest, we performed a sensitivity analysis limited to individuals who did not have a diagnosis of asthma at age 6. This did not affect the relationship between high insulin and subsequent asthma (Table 2).
Analyses in ALSPAC
Fasting insulin levels were measured at age 8 in serum samples for 857 subjects who had at least one asthma assessment between ages 8 and 17. Participants included in this analysis (n=857) were similar to those excluded for lack of insulin measurement and asthma information (n=37) except that included participants had slightly older mothers (see table e3 in the Online Repository).
Fasting insulin levels at age 8 were higher in females (n=403) compared to males (n=454) (median, interquartile range [in uIU/ml]; 5.3(3.5, 8.5) vs 4.1 (2.9, 7.1), respectively, p<0.0001 by Kruskall-Wallis). We defined high insulin and low insulin groups for ALSPAC as described in the methods. There were no differences in maternal characteristics or early life exposures between participants with high insulin compared to those with low insulin at age 8 (Table 3).
Table 3.
ALSPAC: Characteristics of study participants by age 8 high and low insulin group. Number of participants in each group are noted in the table when they differ from the overall insulin groups.
| Characteristics | Group | Low Insulin (N=641) |
High Insulin (N=216) |
p | ||
|---|---|---|---|---|---|---|
| % | n | % | n | |||
| Sex | Male | 53.0 | 340 | 52.8 | 114 | 0.946 |
| Ethnic background | White | 97.1 | 606/624 | 98.1 | 205/209 | 0.619 |
| Non-White | 3.0 | 18/624 | 2.0 | 4/209 | ||
| Maternal asthma | Yes | 14.6 | 91/624 | 14.4 | 30/208 | 0.955 |
| Maternal smoking, last 2 months of pregnancy | Yes | 11.8 | 74/630 | 13.2 | 28/211 | 0.557 |
| Maternal age at delivery | <20 years | 1.4 | 9/641 | 1.4 | 3/216 | |
| 20–36 years | 92.0 | 590/641 | 92.6 | 200/216 | ||
| >36 years | 6.6 | 42/641 | 6.0 | 13/216 | 0.967 | |
| Ever breastfed in the first 6 months | Yes | 84.3 | 525/623 | 83.9 | 177/211 | 0.895 |
| Day care attendance during the first year | Yes | 8.9 | 55/616 | 7.0 | 14/201 | 0.385 |
| Pets ownership during the first year | Yes | 67.3 | 417/620 | 70.8 | 143/202 | 0.349 |
| Maternal smoking at 8 months after birth | Yes | 16.4 | 102/622 | 15.9 | 33/207 | 0.877 |
| Characteristics, age 8 | ||||||
| BMI 1 , UK z-score (SD) | Mean (95%CI) | 0.21 (0.13, 0.28) | 637 | 0.73 (0.59, 0.88) | 216 | <0.001 |
| BHR 1 | Yes | 15.3 | 94/613 | 14.4 | 29/201 | 0.946 |
| Asthma | Yes | 14.5 | 80/553 | 22.5 | 41/182 | 0.011 |
| Skin test positivity | Yes | 23.0 | 131/569 | 22.7 | 44/194 | 0.922 |
| Total IgE, IU/mL | GM (95%CI) | 72.0 (62.0, 83.6) | 483 | 84.4 (65.1, 109) | 151 | 0.303 |
BMI included as z-scores from UK reference tables; BHR included as highest tertile with positive methacholine challenge(40)
Abbreviations: BMI - body mass index; BHR - bronchial hyper-responsiveness; IgE - immunoglobulin E; GM - geometric mean
Children in the high insulin group were more likely to have asthma at age 8 (22.5% [n=41/182] vs 14.5% [n=80/553], p=0.011), and to have a higher mean BMI compared to those with low insulin (18.1 [95%CI 17.7, 18.6] vs. 16.7 [16.6, 16.9], p<0.001) (Table 3). Fasting insulin levels at age 8 were unrelated to total IgE (p=0.303), positive skin testing at age 7 (p=0.922), or methacholine responsiveness (p=0.946).
High insulin at age 8 was significantly associated with active asthma between ages 8 and 17 years compared to low insulin, when assessed in cross-sectional analyses (Figure 2). In a longitudinal model, high insulin was associated with a significantly higher risk of active asthma from ages 11 to 17 compared to low insulin (1.59 [1.12–2.27], p=0.009, n=814; Table 4). The relation between high insulin and active asthma remained significant after adjustment for zBMI and sex (OR 1.51 [1.05–2.17], p=0.026, n=811), while that between zBMI and asthma was not significant (OR 1.10 [0.94–1.29), p=0.243). High insulin was also associated with active asthma in a sensitivity analysis limiting the model to those with no asthma at age 8 (OR 2.14 [1.10–4.17], p=0.026; n=584) and adjusted for zBMI and sex. Effect estimates were not substantially changed in models adjusted for serum triglycerides, leptin and CRP (see Table e4 in the Online Repository).
Figure 2.
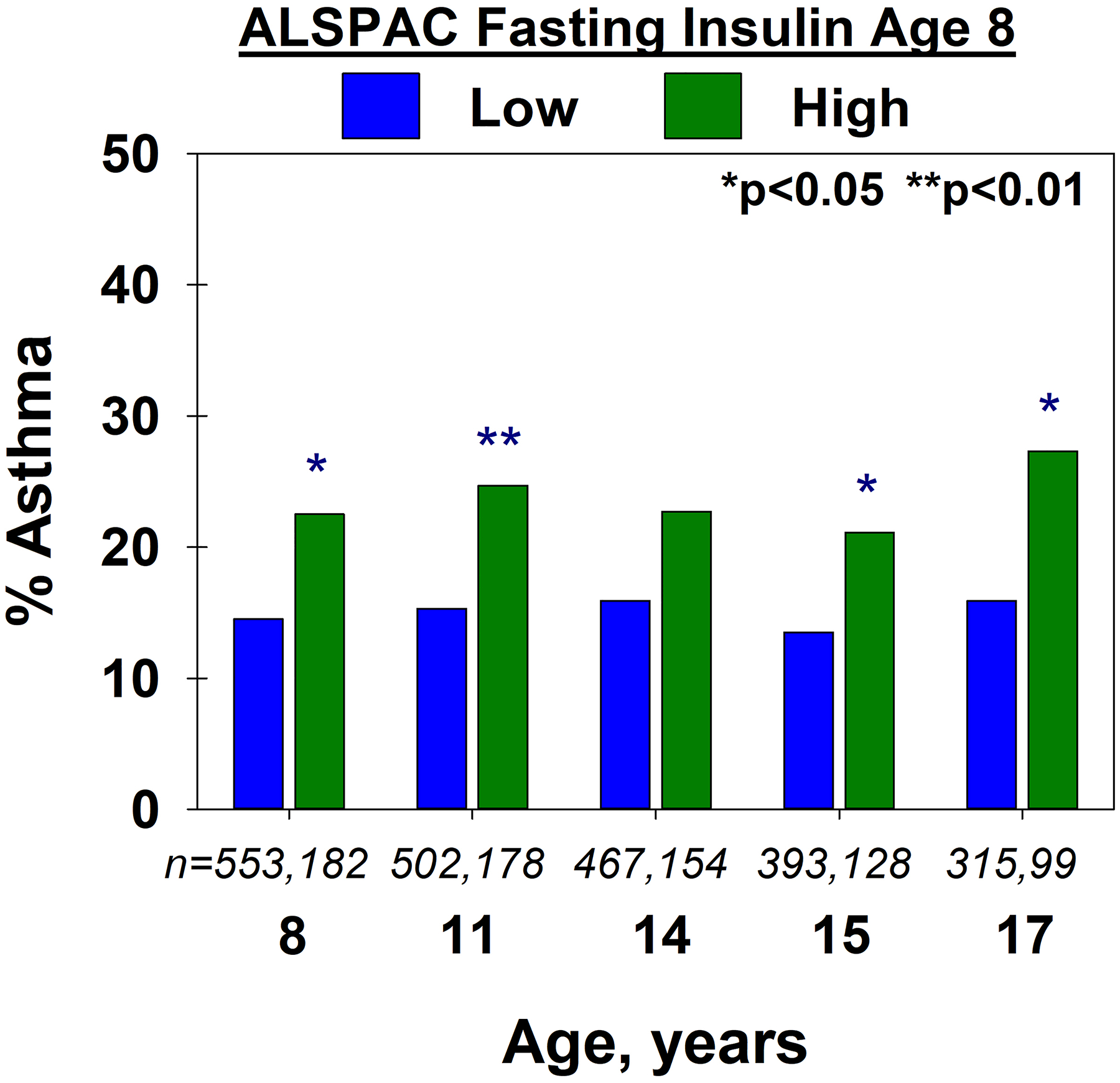
ALSPAC: The proportion of participants with asthma at each age, by low vs. high fasting insulin, in the ALSPAC cohort. Number of participants in the low and high insulin groups are shown in italics
Table 4.
ALSPAC: Odds of subsequent asthma ages 11–17 years, comparing high fasting insulin with low fasting insulin at age 8. All models adjusted for sex; models 2 and 3 adjusted for z-score BMI1 at age 8. Models 1 and 2 include all participants, model 3 is limited to those with no asthma at age 8.
| Models | Odds Ratio | 95% CI | p | n | Observations | |
|---|---|---|---|---|---|---|
| 1 | All participants | |||||
| High Fasting Insulin | 1.59 | 1.12, 2.27 | 0.009 | 814 | 2236 | |
| Female | 1.11 | 0.80, 1.54 | 0.521 | |||
| 2 | All participants with BMI | |||||
| High Fasting Insulin | 1.51 | 1.05, 2.17 | 0.026 | 811 | 2236 | |
| Female | 1.11 | 0.80, 1.53 | 0.538 | |||
| zBMI | 1.10 | 0.94, 1.29 | 0.243 | |||
| 3 | Limited to those with no asthma at age 8 | |||||
| High Fasting Insulin | 2.14 | 1.10, 4.17 | 0.026 | 5842 | 1729 | |
| Female | 1.55 | 0.82, 2.95 | 0.180 | |||
| zBMI | 0.99 | 0.72, 1.36 | 0.942 | |||
BMI included as z-scores from UK reference tables
584=811 minus 106 (with diagnosis of asthma at age 8) minus 121 (missing asthma information at age 8)
Fasting insulin at 8 years old tended to be weakly correlated with fasting insulin at age 13 (n=448, r=0.20) and age 17 (n=425, r=0.12). There was no relation between fasting insulin measured at age 13 and subsequent asthma through age 17, nor was there a relation between fasting insulin measured at age 17 and asthma at age 17 (see Table e5 in the Online Repository).
Discussion:
In two independent birth cohorts, higher serum insulin levels at ages 6 or 8 years were associated with active asthma in childhood, adolescence, and adulthood. The effect was independent of concurrent BMI and was not related to other demographic and asthma risk factors nor other tested markers of systemic inflammation and metabolic syndrome. However, there was no relation between fasting insulin during puberty and concurrent or subsequent asthma. These data suggest that during the prepubertal years, insulin may contribute to asthma risk, possibly via pathways directly related to insulin function.
The analyses presented herein compared the highest quartile to lower three quartiles of insulin because no further dose response for insulin was evident in either cohort (see Figures e2, e3 in the Online Repository). In ALSPAC, insulin levels were measured after participants fasted overnight, consistent with the most commonly used method of identifying insulin resistance in children(42). In TCRS, as for most birth cohort studies, non-fasting blood samples were drawn. Although Hancox et al(43) showed that fasting and non-fasting serum insulin levels show only a moderate correlation (r=0.44) in children, the fact that similar associations were found between high serum insulin and asthma risk in ALSPAC and TCRS strongly vouches for the validity of our findings. However, the potential utility of non-fasting testing for metabolic analytes should be acknowledged, for ease of sampling, participant safety, and reflection of actual daily state(44, 45).
A strong association between obesity and asthma is now well established (10, 46, 47), but the mechanisms that explain it remain obscure. Obesity is associated with chronic low-grade systemic inflammation characterized by increased serum levels of TNF-α, IL-1β, and IL-6(48), but there is not current evidence that antagonists for any of these mediators are effective in treating obesity-associated asthma. Obesity increases the risk for a variety of adverse long term health consequences, but asthma outcomes appear to be more strongly associated with metabolic dysfunction than with BMI or body fat mass(3). Among the known markers of metabolic dysfunction, insulin resistance seems to be the most relevant: In the aforementioned National Health and Nutrition Examination Study citation(19) the interaction between obesity and insulin resistance as determinants of asthma prevalence in adults was robust to adjustments for other metabolic syndrome markers, including hypertriglyceridemia and levels of CRP. Bronchial epithelial cells from patients with asthma show evidence of insulin resistance(49), and in a retrospective study in Taiwan involving over 50,000 persons, incidence of asthma was 30% higher among subjects with a diagnosis of diabetes than among those without diabetes, suggesting the importance of insulin in asthma risk(50). Moreover, in a general population cohort of adults in Copenhagen, insulin resistance was associated with increased odds of subsequent development of wheezing or asthma-like symptoms(51). Recently, a cross-sectional analysis of the UK Biobank data supported elevated blood glucose levels as measured by hemoglobin A1C as a risk for asthma-related hospitalizations(52). We extend these observations herein, showing that merely increased levels of insulin in early childhood predict subsequent asthma into adulthood. These data imply that the mechanism underlying insulin-associated asthma risk is neither solely mediated by obesity nor dependent on the metabolic syndrome, and instead may be due to a direct, still undefined biological effect of insulin itself.
Age related effects are notably important in the relation of high insulin to asthma. The relation of age 6 insulin to asthma was not present at age 6 in TCRS, but was evident by age 8 in both TCRS and ALSPAC cohorts. Further, the relationship between increased serum insulin and risk for asthma was limited to insulin measured in the pre-pubertal period. These findings are in agreement with previous reports indicating that insulin measured during adolescence is unrelated to asthma(53, 54). The discrepancy between asthma risk related to measurements of pre-pubertal versus peri-pubertal insulin may reflect the marked changes in insulin serum concentrations subsequent to concurrent growth hormone surges and decreased insulin sensitivity during puberty(55, 56).
Animal and ex-vivo human studies have indicated potential mechanisms by which excessive insulin may directly contribute to asthma development. In a murine model, exogenous intranasal insulin administration to mice increased lung beta-catenin levels, airway hyperreactivity, and collagen deposition in the lungs, with associated proliferation of procontractile and profibrotic smooth muscle cells(57). Perhaps most relevant to our findings are experiments performed by Nie et al(58). They showed that obesity increased bronchial responsiveness elicited by stimulation of the vagus nerve, but not by administration of a parasympathetic stimulant, acetylcholine. Further, this effect was blocked by the drug streptozocin, which reduces insulin secretion from beta cells in the pancreas, and the effect was restored by insulin administration. Evidence was also presented suggesting that increased insulin mediates bronchial responsiveness by inducing a loss of presynaptic M2 muscarinic (inhibitory) receptor function in both obesity-prone and obesity-resistant rats. Interestingly, we found a significant correlation between insulin levels and bronchial hyper-responsiveness induced by cold, dry air challenge in TCRS, which has a neural component(59), but not by methacholine in ALSPAC. This observation suggests that the mechanisms through which insulin may elicit bronchial hyper-responsiveness may be different from those present in allergic forms of asthma associated with positive responses to methacholine, a speculation consistent with the lack of association between insulin levels and markers of an allergic diathesis in both cohorts.
Our results suggest the tantalizing possibility that therapeutic strategies that decrease insulin levels or address insulin resistance in early life may contribute to the prevention of asthma. Nutritional approaches that limit weight gain or support healthy metabolism between birth and three years could decrease the incidence of insulin resistance and thus of asthma(6). Interestingly, Chen et al showed that Taiwanese diabetic patients treated with metformin were less likely to develop asthma than those treated with insulin(50). A claims-based analysis supported these data, showing that metformin therapy is associated with lower hazard for severe asthma exacerbations(60). It is therefore tempting to speculate that treatment with metformin may decrease incidence of asthma in young children with high insulin levels, while acknowledging that antidiabetic drug trials have previously failed to show benefit for asthma control(61).
Strengths of this study include the remarkable reproducibility and consistency of our findings in two extensively phenotyped, longitudinally studied independent populations. Limitations of these cohorts include the relative paucity of Black/African-American participants, to whom these findings may not be generalizable. In addition, there were a few differences observed between TCRS and ALSPAC subjects included vs excluded from the analyses that could indicate additional threat to generalizability, as described in the text. The RBM measurement of insulin used in TCRS is not a clinically utilized method and, therefore, should not be compared to published clinical reference ranges. We also acknowledge the lack of fasting insulin and abdominal obesity measurement in the TCRS cohort and lack of glucose measurement from which HOMA-IR could be calculated as an alternative marker of insulin resistance. Longitudinal assessment of insulin measurements on asthma risk was limited by lack of insulin data throughout childhood and into adulthood. However, this analysis is likely to be of limited utility in light of the significant effect of puberty on insulin measurements. The definition of atopy by skin testing in both cohorts could contribute to measurement bias through including a limited panel of locally relevant allergens and a conservative wheal size cutoff. Finally, the potential effect of intercurrent asthma therapy is difficult to determine in these cohorts.
In conclusion, higher blood insulin in early childhood is associated with an increased risk for asthma up to adult life, independent of body mass index, and reduced lung function in adulthood. The possible pathophysiologic mechanisms of this association are not yet fully understood but warrant additional study. Higher serum insulin may be a pharmacologic target for prevention of asthma, particularly in early childhood.
Supplementary Material
Highlights Box:
The relationship of asthma to obesity is complex, but strengthened among those with insulin resistance.
We found that, in two birth cohorts, high insulin in childhood is associated with increased risk of asthma development through adolescence and adulthood, independent of body mass index.
This association signals an early life metabolic pathway toward asthma development that may be a modifiable risk factor.
Acknowledgements:
The authors dedicate this work to Dr. John Henderson, who actively participated in the design and conduction of the study.
TCRS Acknowledgements:
The authors gratefully acknowledge the contributions of Lynn Taussig who started the Tucson Children’s Respiratory Study in 1980. We thank the participants and their families; Lily Kim, Bruce Saul and David Spies for data and manuscript management; all lab technicians involved in TCRS since 1980, specifically Amber Spangenberg and Dayna Anderson; and the study nurses, Marilyn Lindell, Lydia de la Ossa, Nicole Pargas and Silvia Lopez for data collection and participant follow-up.
ALSPAC Acknowledgements:
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Funding:
The Tucson Children’s Respiratory Study is funded through the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL132523). The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Dr Martinez will serve as guarantor for the contents of this paper.
Conflict of Interest Statement:
TFC reports consulting fees from AstraZeneca, Genentech, GSK, Novartis, Regeneron and royalties from UpToDate outside the submitted work. SG has received grants from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute, and the NIH/National Institute of Allergy and Infectious Diseases. FDM has received grants from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute, the NIH/National Institute of Allergy and Infectious Diseases, the NIH/Office of the Director, and OM Pharmaceuticals. RG, DAS, AW, MH, JH have no conflicts to disclose.
Abbreviations:
- ALSPAC
Avon Longitudinal Study of Parents and Children
- BHR
Bronchial hyper responsiveness
- BMI
Body mass index
- TCRS
Tucson Childrens’ Respiratory Study
References
- 1.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382(9901):1360–72. [DOI] [PubMed] [Google Scholar]
- 2.Granell R, Henderson AJ, Evans DM, Smith GD, Ness AR, Lewis S, et al. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS medicine. 2014;11(7):e1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, et al. The nonallergic asthma of obesity. A matter of distal lung compliance. American journal of respiratory and critical care medicine. 2014;189(12):1494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marijsse GS, Seys SF, Schelpe AS, Dilissen E, Goeminne P, Dupont LJ, et al. Obese individuals with asthma preferentially have a high IL-5/IL-17A/IL-25 sputum inflammatory pattern. Am J Respir Crit Care Med. 2014;189(10):1284–5. [DOI] [PubMed] [Google Scholar]
- 6.Rastogi D. Pediatric obesity-related asthma: A prototype of pediatric severe non-T2 asthma. Pediatr Pulmonol. 2020;55(3):809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101(11):2240–7. [DOI] [PubMed] [Google Scholar]
- 8.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. [DOI] [PubMed] [Google Scholar]
- 9.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGarry ME, Castellanos E, Thakur N, Oh SS, Eng C, Davis A, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14(3):222–31. [DOI] [PubMed] [Google Scholar]
- 12.Lombardi E, Stern DA, Sherrill D, Morgan WJ, Wright AL, Garcia-Aymerich J, et al. Peak flow variability in childhood and body mass index in adult life. J Allergy Clin Immunol. 2019;143(3):1224–6 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Tu YK, Huang KC, Chen PC, Chu DC, Lee YL. Pathway from central obesity to childhood asthma. Physical fitness and sedentary time are leading factors. Am J Respir Crit Care Med. 2014;189(10):1194–203. [DOI] [PubMed] [Google Scholar]
- 14.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014;19(8):1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758–69. [DOI] [PubMed] [Google Scholar]
- 16.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–43. [DOI] [PubMed] [Google Scholar]
- 17.Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, et al. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia. 2004;47(6):1064–70. [DOI] [PubMed] [Google Scholar]
- 18.Astley CM, Todd JN, Salem RM, Vedantam S, Ebbeling CB, Huang PL, et al. Genetic Evidence That Carbohydrate-Stimulated Insulin Secretion Leads to Obesity. Clin Chem. 2018;64(1):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardet JC, Ash S, Kusa T, Camargo CA, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48(2):403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661–75; quiz 76. [DOI] [PubMed] [Google Scholar]
- 21.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study. I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129(6):1219–31. [DOI] [PubMed] [Google Scholar]
- 22.Vidmar SI, Cole TJ, Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: Update. The Stata Journal. 2013;13(2):366–78. [Google Scholar]
- 23.Carr TF, Stern DA, Halonen M, Wright AL, Martinez FD. Non-atopic rhinitis at age 6 is associated with subsequent development of asthma. Clin Exp Allergy. 2019;49(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–8. [DOI] [PubMed] [Google Scholar]
- 25.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135(4):607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 27.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372(9643):1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern DA, Lohman IC, Wright AL, Taussig LM, Martinez FD, Halonen M. Dynamic changes in sensitization to specific aeroallergens in children raised in a desert environment. Clin Exp Allergy. 2004;34(10):1563–669. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi E, Morgan WJ, Wright AL, Stein RT, Holberg CJ, Martinez FD. Cold air challenge at age 6 and subsequent incidence of asthma. A longitudinal study. Am J Respir Crit Care Med. 1997;156(6):1863–9. [DOI] [PubMed] [Google Scholar]
- 31.Martinez FD, Stern DA, Wright AL, Taussig LM, Halonen M. Association of non-wheezing lower respiratory tract illnesses in early life with persistently diminished serum IgE levels. Group Health Medical Associates. Thorax. 1995;50(10):1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golding J, Pembrey M, Jones R, Team AS. ALSPAC--the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. [DOI] [PubMed] [Google Scholar]
- 33.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42(1):111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granell R, Henderson AJ, Sterne JA. Associations of wheezing phenotypes with late asthma outcomes in the Avon Longitudinal Study of Parents and Children: A population-based birth cohort. J Allergy Clin Immunol. 2016. [Google Scholar]
- 36.Oksel C, Granell R, Mahmoud O, Custovic A, Henderson AJ. Causes of variability in latent phenotypes of childhood wheeze. J Allergy Clin Immunol. 2018. [Google Scholar]
- 37.Ong KK, Elmlinger M, Jones R, Emmett P, Holly J, Ranke MB, et al. Growth hormone binding protein levels in children are associated with birth weight, postnatal weight gain, and insulin secretion. Metabolism. 2007;56(10):1412–7. [DOI] [PubMed] [Google Scholar]
- 38.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souef PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6(7):526–34. [DOI] [PubMed] [Google Scholar]
- 39.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–29. [DOI] [PubMed] [Google Scholar]
- 40.Elliott L, Henderson J, Northstone K, Chiu GY, Dunson D, London SJ. Prospective study of breast-feeding in relation to wheeze, atopy, and bronchial hyperresponsiveness in the Avon Longitudinal Study of Parents and Children (ALSPAC). J Allergy Clin Immunol. 2008;122(1):49–54, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson J, Sherriff A, Farrow A, Ayres JG. Household chemicals, persistent wheezing and lung function: effect modification by atopy? Eur Respir J. 2008;31(3):547–54. [DOI] [PubMed] [Google Scholar]
- 42.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. [DOI] [PubMed] [Google Scholar]
- 43.Hancox RJ, Landhuis CE. Correlation between measures of insulin resistance in fasting and non-fasting blood. Diabetol Metab Syndr. 2011;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langsted A, Nordestgaard BG. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. 2019;51(2):131–41. [DOI] [PubMed] [Google Scholar]
- 45.Darras P, Mattman A, Francis GA. Nonfasting lipid testing: the new standard for cardiovascular risk assessment. CMAJ. 2018;190(45):E1317–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen F, Hancox RJ. Mechanisms of obesity in asthma. Curr Opin Allergy Clin Immunol. 2014;14(1):35–43. [DOI] [PubMed] [Google Scholar]
- 47.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121(5):1087–93; quiz 94–5. [DOI] [PubMed] [Google Scholar]
- 48.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54(6):945–55. [DOI] [PubMed] [Google Scholar]
- 49.Loffredo LF, Abdala-Valencia H, Anekalla KR, Cuervo-Pardo L, Gottardi CJ, Berdnikovs S. Beyond epithelial-to-mesenchymal transition: Common suppression of differentiation programs underlies epithelial barrier dysfunction in mild, moderate, and severe asthma. Allergy. 2017;72(12):1988–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CZ, Hsu CH, Li CY, Hsiue TR. Insulin use increases risk of asthma but metformin use reduces the risk in patients with diabetes in a Taiwanese population cohort. J Asthma. 2017;54(10):1019–25. [DOI] [PubMed] [Google Scholar]
- 51.Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39(5):700–7. [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Han YY, Forno E, Yan Q, Rosser F, Chen W, et al. Glycated Hemoglobin A1c, Lung Function, and Hospitalizations Among Adults with Asthma. J Allergy Clin Immunol Pract. 2020;8(10):3409–15 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forno E, Han YY, Muzumdar RH, Celedon JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136(2):304–11 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozyrskyj AL, Zeng Y, Saurek-Aleksandrovska N, Sellers EA, Ramsey CD, Becker AB. Insulin resistance, puberty, and nonatopic asthma in adolescent girls. Am J Respir Crit Care Med. 2014;190(4):474–7. [DOI] [PubMed] [Google Scholar]
- 55.Hindmarsh P, Di Silvio L, Pringle PJ, Kurtz AB, Brook CG. Changes in serum insulin concentration during puberty and their relationship to growth hormone. Clin Endocrinol (Oxf). 1988;28(4):381–8. [DOI] [PubMed] [Google Scholar]
- 56.Kelsey MM, Zeitler PS. Insulin Resistance of Puberty. Curr Diab Rep. 2016;16(7):64. [DOI] [PubMed] [Google Scholar]
- 57.Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol. 2016;310(9):L837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51(2):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heaton RW, Henderson AF, Gray BJ, Costello JF. The bronchial response to cold air challenge: evidence for different mechanisms in normal and asthmatic subjects. Thorax. 1983;38(7):506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of Metformin Initiation and Risk of Asthma Exacerbation. A Claims-based Cohort Study. Ann Am Thorac Soc. 2019;16(12):1527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dixon AE, Subramanian M, DeSarno M, Black K, Lane L, Holguin F. A pilot randomized controlled trial of pioglitazone for the treatment of poorly controlled asthma in obesity. Respir Res. 2015;16:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


