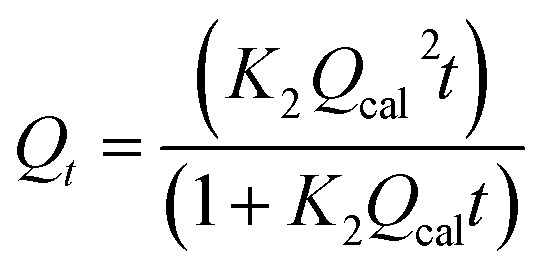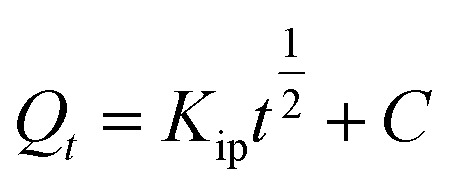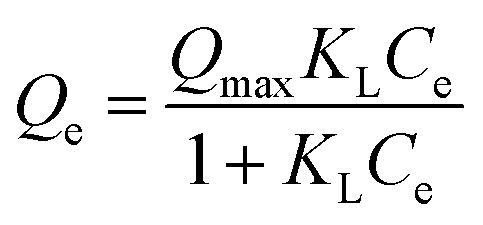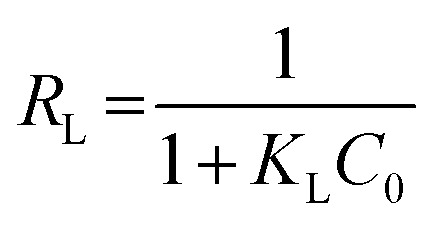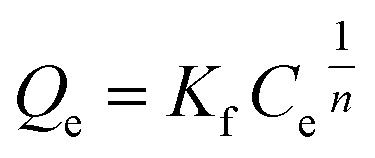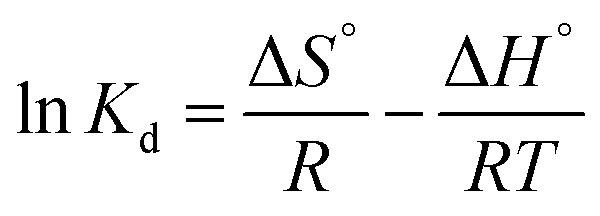|
Q
t
= Qcal(1 − exp K1t) |
Pseudo-first-order (PFO) |
k
1 (min−1): the PFO rate constant; t (min): the contact time of adsorbent and adsorbate; Qt (mg g−1): the amount of paraquat adsorbed at time t; Qcal (mg g−1): the calculated amount of paraquat adsorbed at equilibrium |
38
|
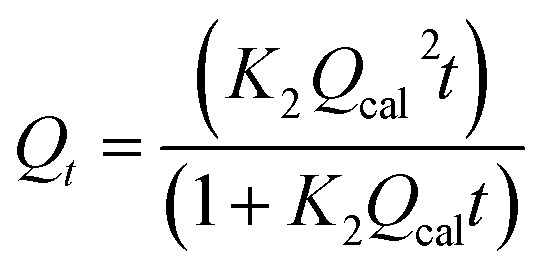
|
Pseudo-second-order (PSO) |
K
2: rate constant |
40
|
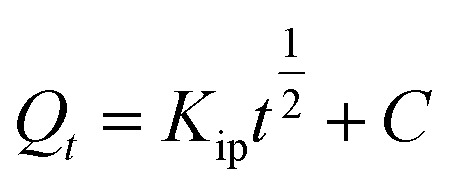
|
Intraparticle diffusion (IPD) |
K
ip (mg g−1 min−1/2): rate coefficient; C (mg g−1): thickness of the boundary layer |
41
|
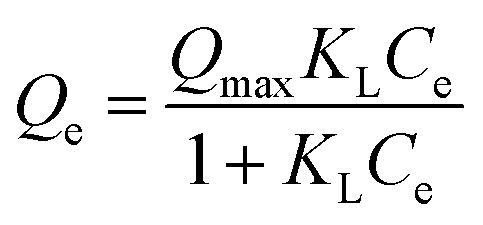
|
Langmuir model |
Q
e (mg g−1) is the equilibrium paraquat or paraquat sorption amount, Ce (mg L−1) is the equilibrium concentration of paraquat or paraquat, 1/n is the Freundlich exponent, Qmax is the maximum adsorbed amount for monolayer sorption, KF ((mg g−1)(mg L−1)−1/n) and KL (mg L−1) represent the Freundlich affinity coefficient and the Langmuir bonding term related to interaction energies, respectively |
42
|
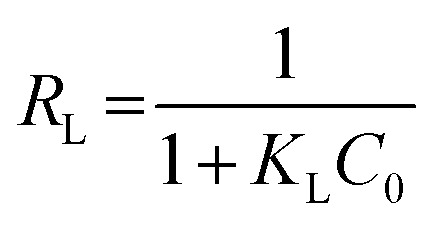
|
Adsorption feasibility |
43
|
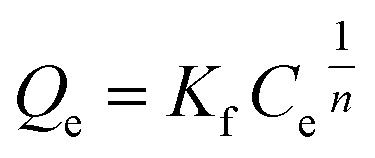
|
Freundlich model |
44
|
| ΔG° = −RT ln Kd
|
Gibbs free energy |
ΔG°: Gibbs free energy change; Kd: equilibrium constant; R: gas constant; T: temperature |
45
|
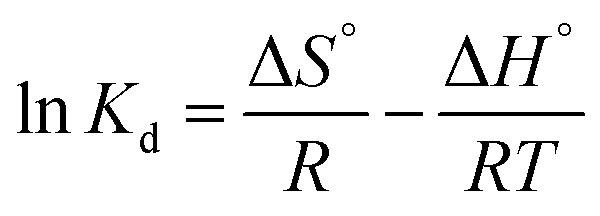
|
Van't Hoff |
ΔS°: entropy change; ΔH°: enthalpy change |
46
|