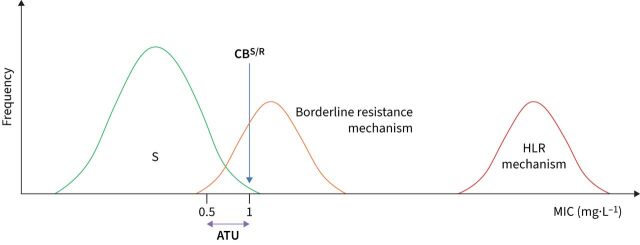
FIGURE 2.
Strategies to minimise false-susceptible results by phenotypic antimicrobial susceptibility testing (AST) linked to borderline resistance mechanisms. Unlike the idealised scenario depicted in figure 1a, borderline resistance mechanisms exist with minimum inhibitory concentration (MIC) distributions that overlap with the susceptible distribution (e.g. the seven borderline rifampicin resistance mutations in rpoB) [10, 13, 37–39]. A clinical breakpoint (CBS/R) that corresponds to the epidemiological cut-off value (ECOFF) (case C in figure 1b) intersects the MIC distributions of such mechanisms (at 1 mg·L−1 in the hypothetical example below). Even if such an isolate is tested multiple times in the same laboratory, it will variably test susceptible and resistant because of the inherent technical variability of phenotypic AST [18]. Four measures that are not mutually exclusive can be taken to decrease such false-susceptible results. First, the optimal solution would be to eliminate or at least minimise the degree of overlap between distributions by reducing the technical variability of MIC testing as much as possible, which was one of the reasons that prompted the European Committee on Antimicrobial Susceptibility Testing (EUCAST) to develop its reference method and associated procedures to improve quality control [8, 9, 40, 41]. Second, EUCAST has introduced areas of technical uncertainty (ATUs) [24]. In this example, an MIC result of ≤0.5 mg·L−1 would be reported as susceptible, whereas MICs of >1 mg·L−1 would be resistant. By contrast, an MIC result of 1 mg·L−1 would be “uncertain” as the isolate in question could not be unequivocally classified as either susceptible or resistant based on the single MIC result because of the overlapping MIC distributions (i.e. this applies to the borderline resistance mechanism but not high-level resistance (HLR) mechanism) [18]. Although the prevalence of borderline resistance in a particular setting can give an indication of which of these possibilities is more likely, other experimental results are needed to resolve this situation conclusively. For example, if the molecular basis of the borderline resistance mechanism is known and is detected, the isolate could be reported as resistant (i.e. a composite reference standard is used, as WHO recommends for rifampicin) [18, 19, 23]. In fact, the Clinical and Laboratory Standards Institute (CLSI) has set an “inconclusive” category for ethambutol for the Sensititre MYCOTB plate by Thermo Fisher Scientific, which appears to serve as an ATU to minimise false susceptibility due to embB mutations [37, 39]. Third, adopting interpretative reading, whereby the results of two antibiotics that share at least one resistance mechanism are analysed together, may be useful (e.g. if the MICs for bedaquiline and clofazimine are equal to or just above the CBS/R, it is likely that the isolate in question has an Rv0678 mutation) [38]. Finally, a surrogate agent could be tested that provides a better resolution between the relevant distribution (e.g. CLSI and EUCAST recommend pefloxacin as a surrogate for fluoroquinolone resistance in Salmonella enterica) [42].