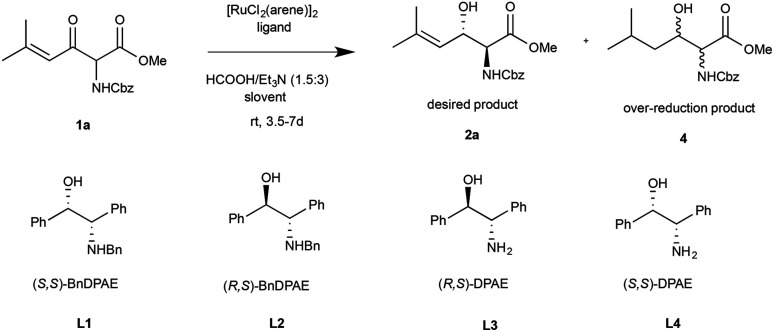
Optimization of the ATH Reaction of 1aa.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Ru dimer | Ligand | Solvent | 1a/2a/4 (%)b | dr (anti : syn)c | er (anti)d |
| 1 | Benzene | L1 | CH2Cl2 | 5/50/20 | 55 : 45 | 88.5 : 11.5 |
| 2 | Benzene | L2 | CH2Cl2 | 21/41/16 | 89 : 11 | 7 : 93 |
| 3 | Benzene | L3 | CH2Cl2 | 35/15/5 | 87 : 13 | 12.5 : 87.5 |
| 4 | Benzene | L4 | CH2Cl2 | <5/52/20 | 80 : 20 | 95 : 5 |
| 5 | p-Cymene | L4 | CH2Cl2 | 33/47/<5 | 85 : 15 | 90.5 : 9.5 |
| 6 | Mesitylene | L4 | CH2Cl2 | 20/65/<5 | 91.5 : 8.5 | 93 : 7 |
| 7 | Hexamethylbenzene | L4 | CH2Cl2 | 34/28/<5 | 83 : 17 | 58 : 42 |
| 8 | Mesitylene | L4 | MeOH | 49/24/<5 | 86 : 14 | 89.5 : 10.5 |
| 9 | Mesitylene | L4 | CH3CN | 26/37/<5 | 85 : 15 | 85 : 15 |
| 10 | Mesitylene | L4 | Dioxane | 8/68/<5 | 93 : 7 | 95.5 : 4.5 |
| 11 | Mesitylene | L4 | CHCl3 | 13/57/<5 | 94.5 : 5.5 | 96 : 4 |
| 12e | Mesitylene | L4 | Dioxane | 11/68/<5 | 93 : 7 | 96 : 4 |
| 13f | Mesitylene | L4 | Dioxane | 15/60/<5 | 94 : 6 | 96 : 4 |
| 14g | Mesitylene | L4 | Dioxane | 12/72/<5 | 95 : 5 | 96.5 : 3.5 |
Reactions preformed with [RuCl2(arene)]2 (0.1 eq.) and ligand (0.2 eq.) heated in 2-propanol (0.3 mL) at 80 °C for 1 h. After cooling to room temperature, the catalyst was then added to a solution of 1a (0.2 mmol, 1 eq.) and HCO2H/Et3N (1.5 : 3, 1.5 eq.) in solvent (1 mL).
Isolated yields.
Determined by NMR analysis of the crude reaction mixture.
Determined by chiral HPLC.
Reaction run with 0.075 eq. of [RuCl2(mesitylene)]2 and 0.15 eq. of (S,S)-DPAE.
Reaction run with 0.05 eq. of [RuCl2(mesitylene)]2 and 0.1 eq. of (S,S)-DPAE.
Reaction run with 0.025 eq. of [RuCl2(mesitylene)]2 and 0.05 eq. of (S,S)-DPAE.
