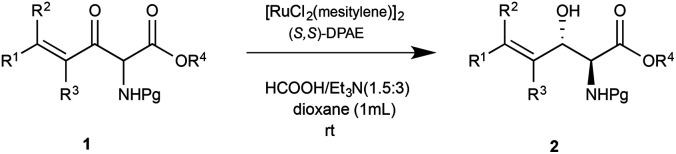
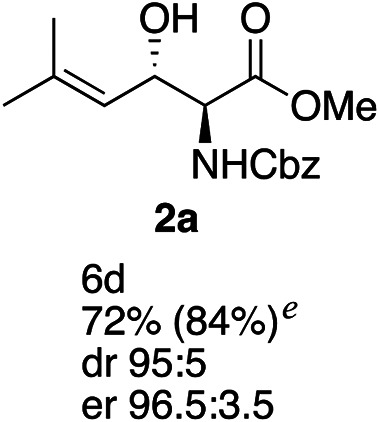
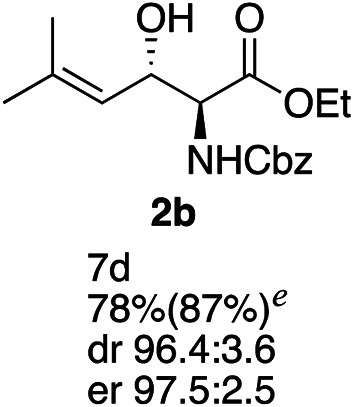
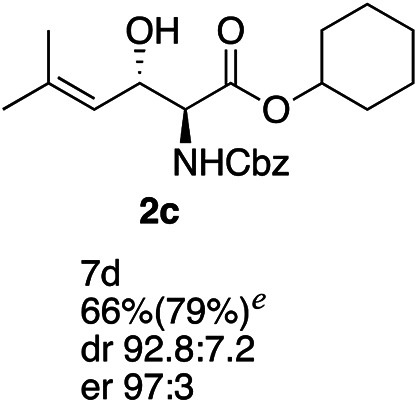
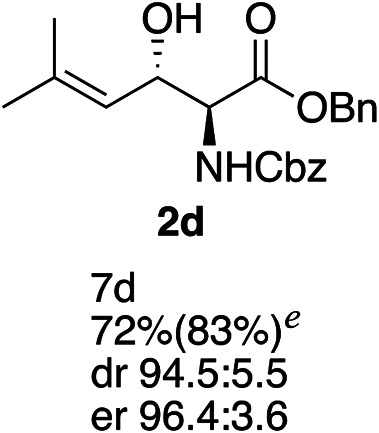
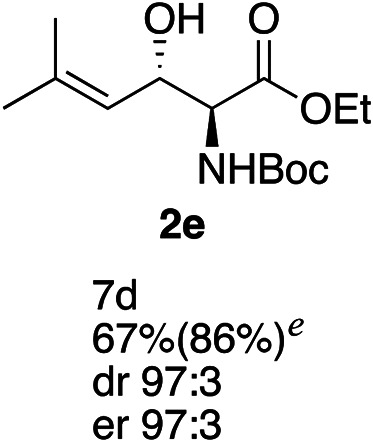
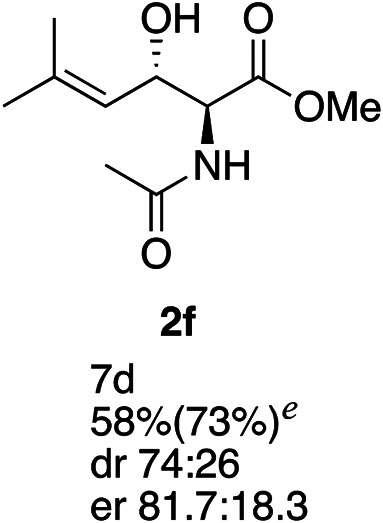
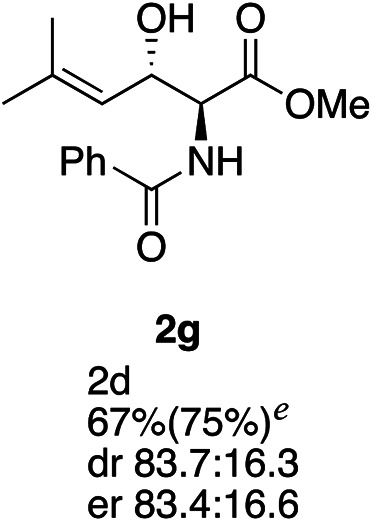
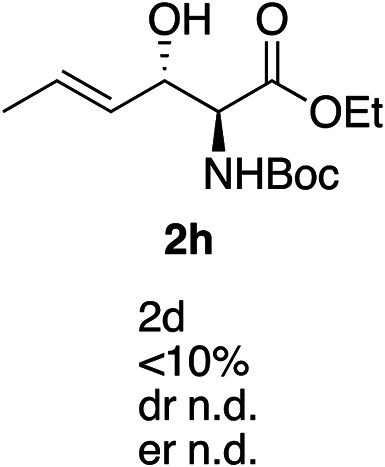
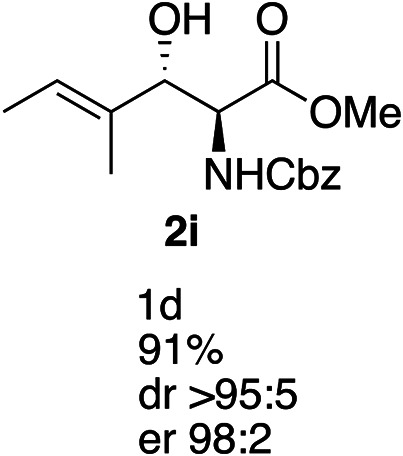
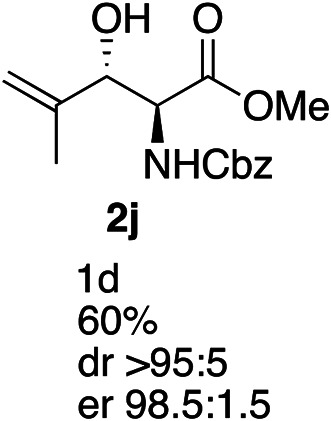
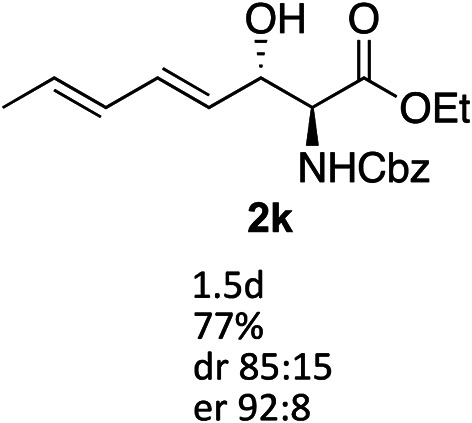
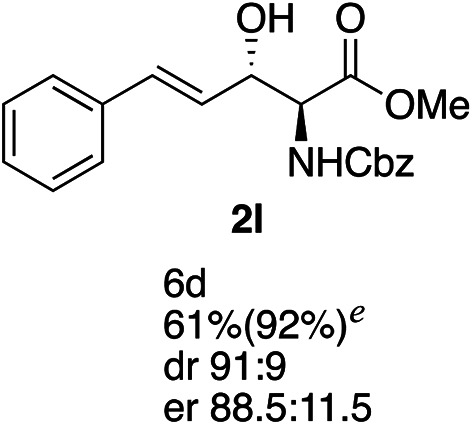
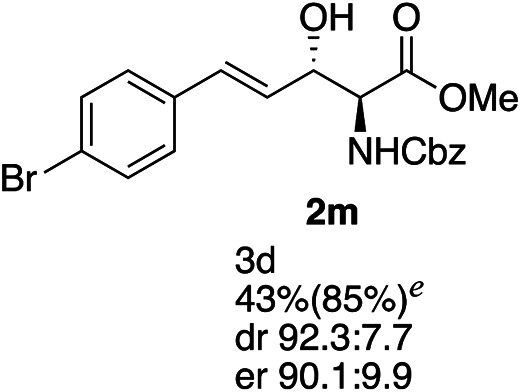
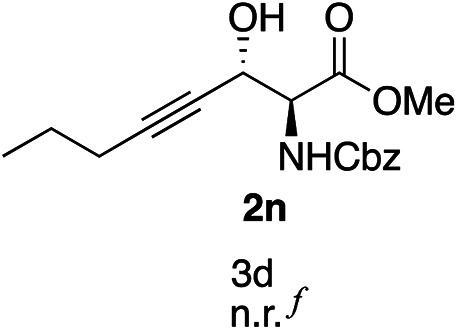
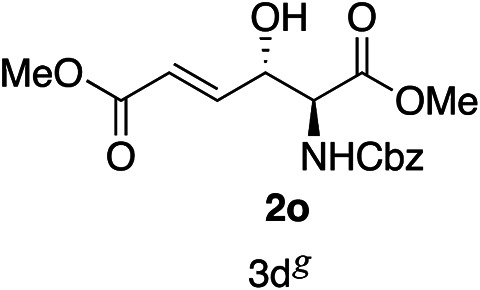
Exploration of substrates scopea,b,c,d.
| ||
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reaction preformed with [RuCl2(mesitylene)]2 (0.025 eq.) and (S,S)-DPAE (0.05 eq.) heated in 2-propanol (0.3 mL) at 80 °C for 1 h. After cooling to room temperature, the catalyst was then added to the β-keto ester 1 (0.2 mmol, 1 eq.) and HCO2H/EtN3 (1.5 : 3, 1.5 eq.) in dioxane (1 mL).
Isolated yields.
dr determined by 1H NMR analysis of the crude reaction mixture.
er determined by chiral HPLC.
Isolated yields based on recovered start material.
No reaction.
The reaction gave a complicated mixture of product.
