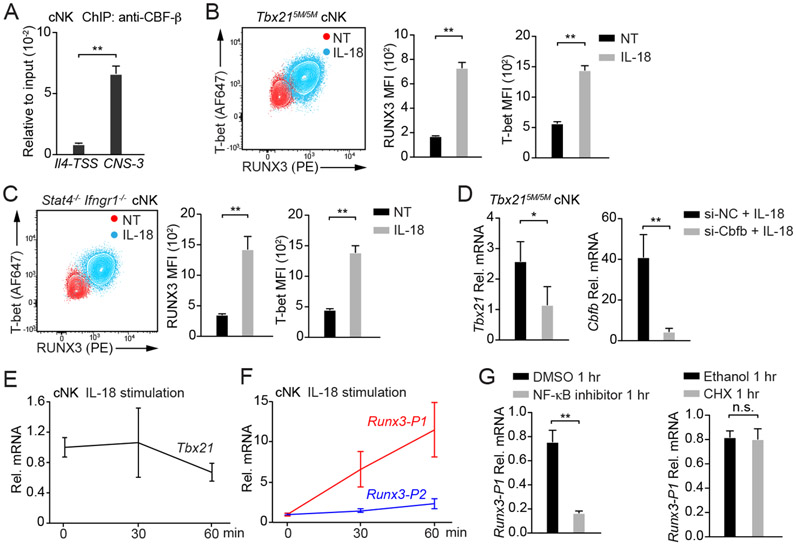
Figure 7. IL-18 induces RUNX3 expression in cNK cells.
(A) Anti-CBF-β ChIP-PCR was performed using cNK cells sorted ex vivo. Primer pairs targeting to Tbx21-CNS-3 and the Il4 transcription start site (Il4-TSS, negative control) were used. Mean ± SD, n=3.
(B and C) Tbx215M/5M cNK cells (n=3) and Stat4−/−Ifngr1−/− cNK cells (n=3) were incubated with or without IL-18 for 3 days in the presence of IL-2. T-bet and RUNX3 protein amounts were measured, and MFI was calculated. Mean ± SD.
(D) Tbx215M/5M cNK cells were co-transfected with siRNA targeting to Cbfb (n=3) or negative control (NC, n=3) and Green indicator by using Amaxa™ 4D-Nucleofector™. After 24 hours, the Greenhi population was sorted and cultured for additional 12 hours. Cells were then incubated with IL-18 and IL-2 for 2 days. The transcripts of Tbx21 and Cbfb were assessed by qRT-PCR. Mean ± SD.
(E and F) cNK cells were stimulated with IL-18 for various time points as indicated. The relative Tbx21 (E), Runx3-P1 (distal) and Runx3-P2 (proximal) mRNA (F) were measured by using qRT-PCR and normalized to Hprt mRNA. Mean ± SD, n=3.
(G) cNK cells were pre-treated with NF-κB inhibitor or cycloheximide (CHX) for 10 minutes and stimulated with IL-18 for 1 hour. The relative Runx3-P1 mRNA was measured. Mean ± SD, n=3.
Data are representative of two (A-G) independent experiments. See also Figure S7 and Table S3.