Abstract
Maternal obesity is an important risk factor for childhood obesity and influences the prevalence of metabolic diseases in offspring. As childhood obesity is influenced by postnatal factors, it is critical to determine whether children born to women with obesity during pregnancy show alterations that are detectable at birth. Epigenetic mechanisms such as DNA methylation modifications have been proposed to mediate prenatal programming. We investigated DNA methylation signatures in male and female infants from mothers with a normal Body Mass Index (BMI 18.5–24.9 kg/m2) compared to mothers with obesity (BMI≥30 kg/m2). BMI was measured during the first prenatal visit from women recruited into the Ontario Birth Study (OBS) at Mount Sinai Hospital in Toronto, ON, Canada. DNA was extracted from neonatal dried blood spots collected from heel pricks obtained 24 hours after birth at term (total n = 40) from women with a normal BMI and women with obesity matched for parity, age, and neonatal sex. Reduced representation bisulfite sequencing was used to identify genomic loci associated with differentially methylated regions (DMRs) in CpG-dense regions most likely to influence gene regulation. DMRs were predominantly localized to intergenic regions and gene bodies, with only 9% of DMRs localized to promoter regions. Genes associated with DMRs were compared to those from a large publicly available cohort study, the Avon Longitudinal Study of Parents and Children (ALSPAC; total n = 859). Hypergeometric tests revealed a significant overlap in genes associated with DMRs in the OBS and ALSPAC cohorts. PTPRN2, a gene involved in insulin secretion, and MAD1L1, which plays a role in the cell cycle and tumor suppression, contained DMRs in males and females in both cohorts. In males, KEGG pathway analysis revealed significant overrepresentation of genes involved in endocytosis and pathways in cancer, including IGF1R, which was previously shown to respond to diet-induced metabolic stress in animal models and in lymphocytes in the context of childhood obesity. These preliminary findings are consistent with Developmental Origins of Health and Disease paradigm, which posits that adverse prenatal exposures set developmental health trajectories.
Introduction
Obesity is a risk factor for many chronic diseases. Rates of obesity are increasing in adults and children; over 600 million adults and 100 million children worldwide had obesity in 2015 [1]. Rates of obesity correlate among family members, and mothers with obesity tend to have children with obesity [2, 3]. The children of mothers with obesity early in pregnancy have more than twice the risk of obesity between ages 2 and 4 [4], and adiposity at birth predicts adiposity at age 8 [5]. In animal studies, maternal obesity has been shown to result in a number of poor health indicators in offspring, including increased adiposity, insulin resistance, and hyperphasia [6] with sex differences in adult offspring that are independent of high caloric diet consumption in postnatal life [6, 7]. Similar to studies in animal models, findings in humans indicate that maternal obesity influences the prevalence of cardiovascular and metabolic diseases in offspring in a sex-specific manner [8]. In this context, women appear at greater risk of metabolic disease than men, due to in part to the influence of sex-specific genetic and steroid hormone regulatory mechanisms in development [9, 10]. Adverse prenatal exposures are thus recognized as important components of the Developmental Origins of Health and Disease (DOHaD) paradigm, which proposes that exposures in early life set developmental health trajectories [11]. As the prevalence of childhood obesity is also influenced by postnatal factors, including diet and physical activity, it is critical to determine whether the children born to women with obesity during pregnancy show alterations that are detectable at birth.
Epigenetic modifications are proposed mechanisms of prenatal programming. DNA methylation modifications at CpG dinucleotides have been most extensively studied in this context. Specific DNA methylation modifications are required for normal development. They are associated with several key processes, including genomic imprinting and the risk of non-communicable diseases [12]. To our knowledge, only four studies have examined the association between maternal obesity and DNA methylation modifications in whole cord blood [13–16], though modifications associated with offspring sex were not reported, despite known sex differences in prenatal effects on growth and adiposity [8]. For example, male sex hormone testosterone increases in embryonic life [17], with a testosterone surge in the second trimester [18]. The number of X chromosomes alone affects adiposity, where dosage of the X chromosome leads to higher adiposity postnatally in mice [19]. Also, the timing of dynamic DNA modifications such as demethylation is earlier in the paternal than in the maternal genome [20]. We hypothesized that children born to women with obesity during pregnancy show alterations in DNA methylation in whole blood that are detectable at birth and are sex-specific.
In the present study, samples were obtained from an ongoing cohort study, the Ontario Birth Study (OBS), based on the availability of non-self reported maternal BMI measured in the first visit of pregnancy care (~12 weeks of pregnancy). Mothers with obesity and normal weight mothers were matched based on maternal age, parity, and infant sex. Strict exclusion criteria included pregnancy complications previously shown to influence DNA methylation signatures, including diabetes [21, 22] and preterm birth [23], chronic use of glucocorticoids (as in asthma or collagen vascular disease) and treatment with glucocorticoids during pregnancy [24] to minimize the influence of these disease states and associated pharmacological treatments. In the resulting study, we examined genomic loci associated with differential methylation as a function of maternal obesity status in dried blood spots collected at term from neonates of both sexes. We used reduced representation bisulfite sequencing (RRBS) to quantitatively profile DNA methylation modifications of CpG dense regions, where DNA methylation is most likely to influence gene regulation, including CpG islands, promoters, gene bodies, and intergenic regions [25]. We also compared gene-specific differential methylation associated with maternal obesity to those in an existing cohort, the Avon Longitudinal Study of Parents and Children (ALSPAC). Data from this cohort were obtained by 450K microarray, which focuses predominantly on CpGs in RefSeq genes (NM and NR; 98.9% of all UCSC RefGenes) [26]. Therefore, the focus of this comparison was on genes and gene pathways associated with differential methylation identified by our primary analysis.
Materials and methods
Subjects and blood samples
Subjects included in this study were selected from women recruited into the Ontario Birth Study (OBS) at Mount Sinai Hospital in Toronto, ON, Canada [27]. This study was approved by the Mount Sinai Hospital Research Ethics Board (MSH REB# 17-0090-E) and the University of Toronto Research Ethics Boards. All persons gave their informed consent prior to their inclusion in the study. Informed consent was obtained from the mothers and from the mothers on behalf of the minors included in this study. Blood samples were collected on Guthrie cards (Whatman 903) by heel prick 24 hours after birth as a part of routine neonatal screening, with an additional card taken for research purposes. The samples were air-dried for at least 4 hours at room temperature and stored at -80°C. At the time of subject selection, women with obesity were defined as BMI ≥ 30 kg/m2 based on their medical records taken during the first visit of pregnancy care (i.e., ~12 weeks of pregnancy) compared to control women with BMI 18.5–24.9 kg/m2 who were matched by maternal age (within 5 years), parity (multiparous or nulliparous), and infant sex to control for factors associated with birth weight [28, 29]. All pregnancies were singleton and delivered at term (≥ 37 weeks). Chronic use of glucocorticoids (e.g., asthma or collagen vascular disease), treatment with glucocorticoids during pregnancy, diabetes, gestational diabetes, and hypertension were exclusion criteria. The stringent inclusion criteria led to the selection of 20 neonatal dried blood spots from infants born to mothers with obesity and 20 neonatal dried blood spots from infants born to women with a normal weight with equal numbers of male (n = 10/obese and n = 10/non-obese) and females (n = 10/obese and n = 10 non-obese) in each condition from the OBS cohort (total n = 40).
Reduced representation bisulfite sequencing (RRBS)
DNA was extracted from the blood spots using methods described previously [30] with some modifications [31]. Briefly, DNA was extracted from small pieces cut from ½ of a blood spot using Proteinase K lysis and following the manufacturer’s protocols (QIAamp DNA Blood Mini Kit (Qiagen: Cat. #51104)). Only samples with DNA Integrity Numbers (DINS) above 7 were included in the study. We generated RRBS libraries using 100ng dsDNA using MspI restriction enzyme digestion and size selection (RRBS Methyl-Seq System 1–16 (Ovation: Part # 0353)). The RRBS libraries were then sequenced in multiplexes of 10 samples, using a NextSeq500 (Illumina) with a 75bp single end read length (Donnelly Sequencing Centre, University of Toronto, Toronto, ON, Canada).
Differentially methylated regions (DMRs) analysis
Adaptor sequences and low quality reads (q < 30) were trimmed using Trim Galore (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) followed by filtering with NuGEN script, as previously described [31]. Alignment and sorting were performed using Bismark [32] and Samtools [33], respectively. DMRs associated with maternal BMI were investigated using the R package methylKit version 1.14.2 [34]. Read counts below 5x and greater than 99.9% of coverage in each sample were discarded to avoid reads showing PCR bias. Default settings in methylKit were used to calculate the bisulfite conversion rate and the reproducibility of the data [34]. MethylKit tiles the genome into non-overlapping Differentially Methylated Regions (DMRs) of 1kb from a given CpG site. We ensured at least 5X reads per CpG site in each sample, as calling significant DMRs has been shown to be sufficient with 5x reads [35, 36]. P-values for each DMR were calculated using the Fisher’s exact test and then adjusted for multiple testing by calculating Benjamini-Hochberg false discovery rate (FDR) corrected q-values using the SLIM method [37]. We considered regions to be DMRs if they were statistically significant at an FDR < 0.05, contained at least 5 CpG sites and had an absolute difference in DNA methylation that was greater than 5%. DNA methylation differences of at least 5% per DMR have been used in several previous studies, including by our group, as a sensitive approach to detect changes more likely to have biological significance [38–42]. Gene annotation was performed using CompEpitool [43] and human genome assembly hg19.
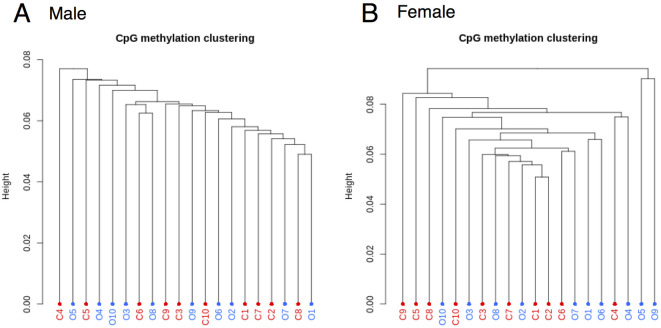
For all reduced representation bisulfite sequencing (RRBS) samples, the bisulfite conversion rate was > 99%. S1 Fig shows a histogram of percent methylation for each sample showing a typical percent methylation histogram with peaks on both ends representing the expected distribution of unmethylated and methylated cytosines using methylKit [34]. As shown in S1 Table, the samples showed high correlations for all pair-wise comparisons (>0.8), demonstrating a high degree of reproducibility in the RRBS dataset [34, 44]. In addition, to enable analyses that were representative of group differences rather than skewing by some samples, we filtered for CpG sites that contained at least 5X reads for each subject [45]. This led to over 1.9 million and 2 million CpG sites for analyses in samples from males and females, respectively. We then performed hierarchical clustering of the samples to examine the similarity in methylation profiles overall. Hierarchical clustering of DNA methylation showed that there was neither grouping by condition (obese, non-obese) nor outliers within each sex (Fig 1).
Fig 1. Hierarchical clustering of samples for males (A) and females (B) in the OBS discovery cohort.
C: neonates of non-obese control mothers (n = 10/sex), O: neonates of mothers with obesity (n = 10/sex).
ALSPAC comparison cohort
Due to the nature of our discovery cohort, which is part of a pilot study, we also examined epigenetic data from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort study to compare differentially methylated genes between cohorts [46, 47]. Women in Avon, UK, during the period of 1st April 1991 and 31st December 1992 were enrolled in the study in early pregnancy, and samples from 14,610 live births were obtained, in accordance with ethical approval from the ALSPAC Ethics and Law Committee and the local research ethics committees. All participants provided informed consent, following ALSPAC Ethics and Law Committee recommendations (see [48] for full data details). There was no involvement of patients or the public in the decision to perform or report this research. In addition, 1018 ALSPAC mother-offspring pairs participated in the Accessible Resource for Integrated Epigenomics Studies (ARIES), which used Illumina Infinium 450k methylation arrays [49].
The same inclusion and exclusion criteria used in the OBS cohort above were applied. There was no difference between obese and non-obese groups for the variables listed in Table 1 for the OBS, except their BMI was taken from pre-pregnancy measures. Samples from the ARIES cohort were derived from cord blood obtained at delivery (n = 1,127 newborn babies). A total of 444 male neonates (n = 16/obese and n = 428/non-obese) and 415 female neonates (n = 13/obese and n = 402/non-obese) for whom we had information on their mother’s pre-pregnancy BMI, were included in our analyses (total n = 859).
Table 1. OBS and ALSPAC subject demographics.
| OBS | ALSPAC | |||||
|---|---|---|---|---|---|---|
| Obese | Control | P value | Obese | Control | P value | |
| # of Infants (male/female) | 20 (10/10) | 20 (10/10) | 29 (16/13) | 830 (428/402) | ||
| Maternal BMI | 36.2 ± 4.8 | 21.6 ± 1.5 | < .01 | 34.7 ± 4.1 | 22.2 ± 2.4 | < 10−16 |
| Maternal age (years) | 33.3 ± 3.5 | 32.8 ± 2.9 | n.s. | 29.1 ± 4.3 | 29.7 ± 4.4 | n.s. |
| Gestational age (weeks) | 39.8 ± 1.0 | 39.5 ± 1.3 | n.s. | 39.6 ± 1.0 | 39.6 ± 1.5 | n.s. |
| Parity (0/1) | 14/6 | 14/6 | n.s. | N/A | N/A | |
| Birthweight | 3571.9 ± 410.6 | 3537.7 ± 466.2 | n.s. | 3683.0 ± 541.1 | 3485.0 ± 457.1 | n.s. |
| Vaginal/C-Section | 10/10 | 15/5 | n.s. | <5/>24 | 45/583 | n.s. |
| (male, female) | (4/6, 6/4) | (8/2, 7/3) | n.s. | N/A | N/A | |
| Medical History (male/female) | ||||||
| Depression* | 4 (2/2) | 1 (1/0) | N/A | N/A | ||
| Anxiety* | 2 (2/0) | 2 (1/1) | N/A | N/A | ||
| Asthma* | 0 (0/0) | 3 (2/1) | N/A | N/A | ||
| Data are presented as mean ± s.d. *not medicated Medical histories are not mutually exclusive Mode of delivery is indicated, where data are available P values were calculated by Chi square test for parity and mode of delivery and t-test for other variables n.s.: not significant N/A: not available | ||||||
RRBS involves restriction enzyme digestion to enrich for areas of the genome with a high CpG content, whereas in 450K arrays, the coverage per gene has a wide range from one to over 1000 CpG sites [50]. As a result, different R packages optimized for identifying DMRs were used following RRBS and array analysis. Preprocessing, filtering of low quality probes, and normalization of 450K Illumina microarray data were performed using the minfi package, version 1.40.0 [51]. DMRs associated with maternal BMI were investigated using the R package DMRcate 2.0.7 [52]. DMRcate fits a limma linear model, adjusted using an empirical Bayes procedure, for each individual CpG site within loci of 1000bp. Default smoothing parameters across individual CpGs within these loci were used, specifically a Gaussian kernel smoother with a bandwidth λ = 1000bp and scaling factor C = 2, with each DMR containing at least 2 CpGs, to account for relative sparsity of coverage by the 450K array in some genomic regions (i.e., intergenic regions) [52]. The resulting kernel-weighted model was compared to the null model using the Satterthwaite method [53] to enable adjustment for multiple testing by FDR. Maximum beta fold-change values per DMR were converted to percentage to summarize effect sizes. As with the sequencing analysis above, DMRs were considered significant if the FDR p < 0.05 with a methylation difference greater than 5% based on the maximum differential methylation value within the DMR. Gene annotation was performed using CompEpitool [43] and the human genome assembly hg19. Hypergeometric tests in R were used to examine the overlap in the number of genes associated with DMRs between the OBS and ALSPAC cohorts.
Gene pathway enrichment analysis
The lists of differentially methylated genes identified by the DMR analyses were explored using MsigDB, a widely used comprehensive database for gene set enrichment analysis [54]. The enrichment analysis was performed using gene sets derived from both the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the biological Gene Ontology (GO) databases, with significant enrichment defined by FDR p < 0.05 using the default background gene set. These tools were used to aid in the interpretation of the biological meaning behind the list of genes associated with DMRs.
Results
Subject demographics are listed in Table 1. There were no differences in incidence of (non-matched) pregnancy complications or other aspects of health-related medical history between the two groups except for maternal BMI which, as expected, was significantly higher in the obese group for both the OBS and ALSPAC cohorts. As there were no differences in incidence of (non-matched) pregnancy complications or other aspects of health-related medical history between the two groups, these factors were not included as covariates.
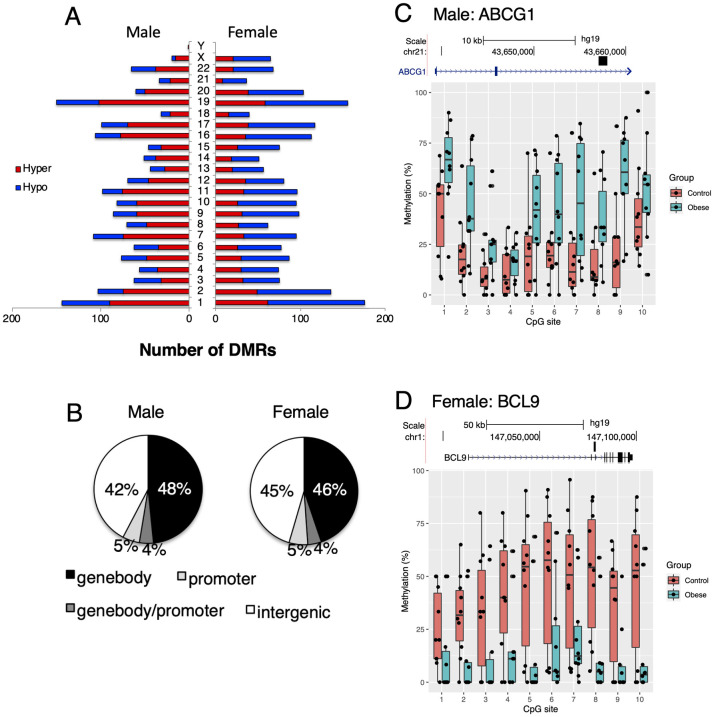
DMRs associated with maternal obesity were found localized to all autosomes as well as in allosomes corresponding to the sex of the offspring (Fig 2A). In male neonates, we identified 1725 differentially methylated regions (DMRs; FDR < 0.05) 1173 regions or 68% were hypermethylated (i.e. greater DNA methylation among neonates from mothers with obesity compared to neonates from mothers with a normal BMI). Five hundred and fifty-two (552) regions or 32% were hypomethylated in male neonates of the mothers with obesity compared to male neonates from mothers with a normal BMI. In female neonates, we identified 2028 DMRs; 710 regions or 35% were hypermethylated and 1318 regions or 65% were hypomethylated compared to females from mothers with a normal BMI. The DMRs identified in male and female neonates were located in genic as well as intergenic loci. Similar numbers of DMRs were localized to intergenic regions in males and females (42% and 45% of all DMRs, respectively). In both sexes, the majority of genic DMRs were found in gene bodies (48% in males and 46% in females). Approximately 9% localized to promoter regions in both sexes (Fig 2B). Examples showing the top differentially methylated DMRs associated with genes in males and females are provided in Fig 2C and 2D. The DMR showing the greatest DNA methylation difference in male neonates was ATP Binding Cassette Subfamily G Member 1 (ABCG1). In female neonates, the top DMRs by percent methylation differences was B-cell CLL/lymphoma 9 protein (BCL9). The full list of gene-annotated DMRs, sorted by absolute methylation difference in percentage, is shown in S2 Table.
Fig 2. Overview of differentially methylated regions in the OBS discovery cohort.
(A) Number of DMRs in relation to chromosomal locations that are either hypermethylated (red) or hypomethylated (blue) in male and female neonates born to mothers with obesity compared to controls. (B) Proportion of DMRs in relation to genetic regions. (C) Representative top DMR in male neonates on the ABCG1 gene showing methylation percentage in individual CpG sites within the DMR. (D) Representative top DMR in female neonates on the BCL9 gene showing methylation percentage in individual CpG sites within the DMR. The genomic track shows the gene location starting from the first exon and the DMR location. Hypermethylation refers to greater DNA methylation among neonates from mothers with obesity compared to neonates from mothers with a normal BMI. Hypomethylation refers to lesser DNA methylation among neonates from mothers with obesity compared to neonates from mothers with a normal BMI.
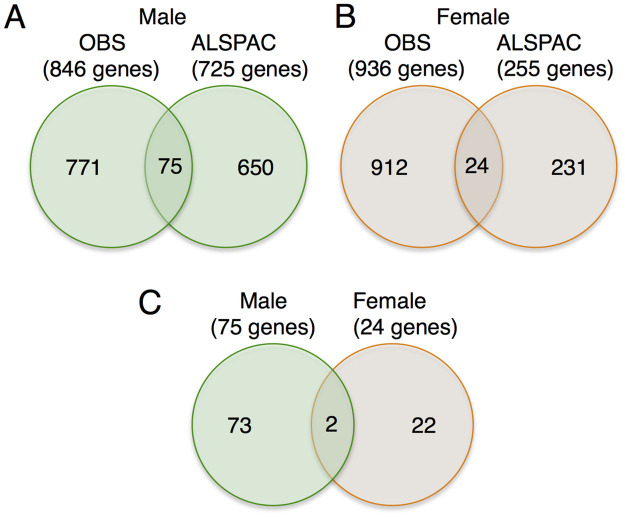
Next, we compared the genes associated with DMRs in the OBS cohort to those of an available population-based cohort, the ALSPAC. The same inclusion and exclusion criteria used in the OBS cohort above were applied. There was no difference between obese and non-obese groups for the variables listed in Table 1 for the OBS, except that BMI was taken from pre-pregnancy measures. In the OBS cohort, male neonates, there were 846 genes associated with DMRs in male neonates and 936 genes associated with DMRs in female neonates (FDRs < 0.05: S2 Table). There were 266 genes common between males and females in the OBS cohort (S2 Table). In the ALSPAC cohort, there were 725 genes associated with DMRs in male neonates and in female neonates, there were 255 genes associated with DMRs (FDRs < 0.05; S3 Table). There were 22 genes common between males and females in the ALSPAC cohort (S3 Table). Among the sex-specific genes, there were 75 genes in common in male neonates and 24 genes in common in female neonates across both cohorts, with 2 genes common to both cohorts and sexes (Fig 3C). Hypergeometric tests revealed significant overlaps in genes associated with DMRs across both cohorts for each sex (P < 10−12 for males and P < 0.001 for females).
Fig 3. Annotation of differentially methylated regions for the OBS and ALSPAC cohorts.
The numbers of genes associated with DMRs for (A) male and (B) female neonates. (C) The number of genes for each sex for genes that replicated across both cohorts. The total number of genes associated with DMRs for each sex are shown in parentheses.
Table 2 shows a list of top genes containing DMRs in both OBS and ALSPAC cohorts, rank-ordered by the total number of DMRs across both cohorts. PTPRN2 (Protein Tyrosine Phosphatase Receptor Type N2) and MAD1L1 (Mitotic Arrest Deficient 1 Like 1) contained DMRs associated with maternal obesity in both sexes in both cohorts. There were 4 and 2 DMRs in PTPRN2 in OBS (methylation difference -12.5 to 6.8%) and ALSPAC (methylation difference 6.0 to 6.3%) male neonates, respectively. In female neonates, PTPRN2 had 5 and 3 DMRs in OBS (methylation difference -12.8 to 10.0%) and ALSPAC (methylation difference 5.9 to 6.7%) respectively. For MAD1L1, there were 3 and 1 DMRs in OBS (methylation difference 5.7 to 6.2%) and ALSPAC (methylation difference 8.4%) male neonates, respectively. In female neonates, MAD1L1 exhibited 1 and 1 DMRs in OBS (methylation difference -5.3%) and ALSPAC (methylation difference 6.8%), respectively. Sex-specific genes in common across both cohorts included the imprinted genes GABRB3 (Gamma-aminobutyric acid receptor subunit beta-3) and PRDM16 (PR/SET Domain 16) in male neonates. S4 Table shows the full list of genes containing DMRs in common between the OBS and ALSPAC cohorts.
Table 2. Top 10 genes associated with differential methylation in neonates, common to the OBS and ALSPAC cohorts.
In some instances, multiple DMRs were associated with a given gene therefore a range of methylation difference percentage is provided. Genes with at least three DMRs are shown. The genes in bold were common to both males and females.
| Male | ||||||||
|---|---|---|---|---|---|---|---|---|
| OBS Meth Diff % | ALS Meth Diff % | |||||||
| Gene | Sum of DMRs (OBS/ALS) | Range min | Range max | [Avg] | Range min | Range max | [Avg] | Function |
| CBFA2T3 | 6 (2/4) | -6.2 | 5.4 | 5.8 | -5.2 | 7.8 | 6.4 | Immune function |
| PTPRN2 | 6 (4/2) | -12.5 | 6.8 | 7.6 | 6.0 | 6.3 | 6.2 | Insulin secretion |
| TCERG1L | 5 (4/1) | 5.0 | 14.5 | 8.6 | 8.2 | 8.2 | 8.2 | Transcription |
| ABL1 | 4 (3/1) | 5.7 | 8.9 | 7.0 | 6.6 | 6.6 | 6.6 | Protooncogene |
| AGAP1 | 4 (3/1) | -7.0 | 10.3 | 8.3 | -7.9 | -7.9 | 7.9 | GTP binding |
| MAD1L1 | 4 (3/1) | 5.7 | 6.2 | 6.0 | 8.4 | 8.4 | 8.4 | Cell cycle |
| PCDHGA1 | 4 (1/3) | 7.4 | 7.4 | 7.4 | 6.1 | 6.7 | 6.3 | Cell adhesion |
| PRDM16 | 4 (1/3) | 5.6 | 5.6 | 5.6 | 5.9 | 7.2 | 6.6 | Immune function |
| RPTOR | 4 (3/1) | 5.4 | 6.7 | 6.2 | 6.6 | 6.6 | 6.6 | Cell growth |
| TPPP | 4 (2/2) | -6.7 | 7.0 | 6.8 | -7.1 | 8.9 | 8.0 | Myelination |
| Female | ||||||||
| PTPRN2 | 8 (5/3) | -12.8 | 10.0 | 8.7 | 5.9 | 6.7 | 6.3 | Insulin secretion |
| BOLA2 | 3 (2/1) | 7.0 | 8.2 | 7.6 | -9.3 | -9.3 | 9.3 | Iron maturation |
| GALNT9 | 3 (2/1) | 5.3 | 7.6 | 6.4 | 9.6 | 9.6 | 9.6 | Oligosaccharide |
| PAX7 | 3 (2/1) | -7.1 | -5.3 | 6.2 | -5.9 | -5.9 | 5.9 | Fetal development |
| RAP1GAP2 | 3 (2/1) | -9.1 | 7.5 | 8.3 | 8.4 | 8.4 | 8.4 | Immune system |
| SEC14L1 | 3 (2/1) | 8.6 | 9.4 | 9.0 | 9.9 | 9.9 | 9.9 | Immune system |
We used gene set functional analysis to biologically contextualize interactions between genes in common between cohorts significantly associated with maternal obesity for each sex. In males, KEGG analysis showed significant enrichment in endocytosis and pathways in cancer (FDRs<0.05). In addition, 33 biological GO pathways in common in both cohorts were significantly enriched in male neonates, primarily involving processes related to cellular signaling, immune system function and development (FDRs <0.05; S5 Table). Biological GO pathways that were significantly enriched among females included “regulation of GTPase activity” and “positive regulation of catalytic activity”, which was also significantly enriched among males (S5 Table; FDRs <0.05). No KEGG pathways were significantly enriched in female neonates, potentially due to the smaller number of genes associated with differential methylation in common to both cohorts.
Discussion
The early life environment during gestation and in infancy is a major factor shaping later life health risk, including susceptibility to the development of obesity. DNA methylation modifications maintain mitotically heritable differences in gene expression in the absence of variations of DNA sequence. Their established role in complex disease and genomic imprinting has led to substantial interest in their potential role as mechanisms mediating the stable programming of health trajectories [55]. Previous studies have largely focused on examining epigenetic differences in offspring by removing data pertaining to sex chromosomes, although there are known sexual dimorphisms in growth and adiposity that occur before birth [8]. In this study, we examined whether DNA methylation modifications associated with maternal obesity in the blood of newborn human infants stratified by offspring sex. We addressed this question by comparing DNA methylation separately in male and female neonatal whole blood from mothers with obesity and compared the identified genes with a large dataset from the ALSPAC cohort. Here we show that DNA methylation profiles in offspring from women with obesity are detectable at birth and they are largely sex-specific.
Using stringent exclusion criteria (excluding common comorbidities associated with maternal obesity such as gestational diabetes and preterm delivery), we found evidence for sex-specific differential DNA methylation and a small of proportion of differentially methylated genes in common to both males and females (~30%) in the OBS cohort. Comparison to the ALSPAC cohort supported these findings, as only a small number of genes were common to both sexes (<10%). Indeed, 75 genes in males and 24 genes in females were common to both cohorts, with only 2 genes common to both sexes across cohorts. Interestingly, IGF1R was differentially methylated in males. The methylation status of IGF1R was previously shown to respond to diet-induced metabolic stress in animal models and was found to be differentially methylated in lymphocytes in the context of childhood obesity [56, 57]. Epigenetic regulation of IGF1R has also been examined in animal models of diabetes. For example, male db/db mice, but not females, show a 7-fold increase in DNA methylation of the Igf1r promotor along with a decrease in levels of Igf1r transcript abundance in skeletal muscle in adulthood, suggesting a sex-specific epigenetic response associated with modifications of gene function in a model of diabetes [58].
Two imprinted genes, namely GABRB3 and PRDM16, also showed differential methylation in both OBS and ALSPAC cohorts in male offspring from mothers with obesity (Table 2; S4 Table). Imprinted genes are implicated in many human disorders, and have important roles in controlling aspects of fetal growth and metabolism [59]. GABRB3 is associated with the pathogenesis of several disorders including Prader-Willi syndrome, the most common genetic cause of morbid obesity in children [60]. Differential methylation of PRDM16 is associated with maternal diabetes in blood of children [61] and in umbilical cord tissue [62], and plays an important role in pancreatic development [63]. PRDM16 is also critical for the differentiation of brown adipose tissue [64], which plays an important role in heat retention and energy expenditure in the first year of life [65] and remains metabolically active into adulthood. Differential methylation of PRDM16 was observed before gastric bypass and weight loss in adipose tissue, suggesting methylation is modifiable by weight loss [66]. DNA methylation in PRDM16 was also found to be reversible with neonatal supplementation with resveratrol in male mice [67]. The presence of differential methylation in blood as well as in primary tissues in these studies highlights the potential for PRDM16 as a biomarker for screening, as well as its potential as a key player in metabolic regulatory mechanisms influenced by maternal obesity. In addition, evidence indicating the responsiveness of PRDM16 to dietary and weight loss intervention suggests it may serve as a molecular target for interventions to mitigate obesity, a hypothesis that deserves further scrutiny.
Among the genes replicated across cohorts in the present study, MAD1L1 and PTPRN2 were common to both sexes (Fig 3; Table 2). For MAD1L1, a gene involved in control of the cell cycle and tumour suppression [68], four DMRs were found in male neonates and two were found in female neonates. There is evidence that methylation levels at the MAD1L1 gene locus are responsive to dietary factors (phytoestrogens [69]). In PTPRN2, a gene implicated in the secretion of insulin with glucose stimuli [70], six and eight DMRs were found in male (4 DMRs in OBS and 2 in ALSPAC) and female neonates (5 DMRs in OBS and 3 in ALSPAC), respectively. Our findings support previous observations of differential methylation in PTPRN2 in newborn blood in association with maternal pre-pregnancy BMI, where analyses were performed with both sexes combined [14]. PTPRN2 encodes a protein that functions as a major islet auto-antigen in type I diabetes [71–73]. Differential methylation in PTPRN2 in whole blood has been associated with gestational diabetes [74–76], with childhood adiposity [76] as well as childhood obesity [77]. The differential methylation in PTPRN2 has also been associated with intrauterine condition such as IUGR in blood [78, 79] but also in adults who have experienced famine, in utero [80]. These findings provide strong evidence that loci in the fetal PTPRN2 are likely modifiable with maternal nutrition, and detectable in blood later in life. Our findings showing differential methylation of these genes in blood at birth suggest they may serve as potential biomarkers of increased risk for developing obesity later in life, though this requires additional study.
As discussed above, several genes identified in this study are genes that are differentially methylated at birth, after birth and in disease states, suggesting their potential roles in the long-term programing by aberrant methylation. Notably, accumulating evidence indicates that the methylation status of these genes may be modifiable, suggesting that interventions in the prenatal or early postnatal period may be beneficial in modifying the trajectory of epigenetic modifications that contribute to subsequent development of metabolic disease. While some known risk factors for childhood obesity, such as low socioeconomic status and excessive food consumption, may differ for males and females in the post-natal period [81, 82], our study suggests that additional consideration of the contribution of prenatal factors is warranted.
Sequencing-based approaches continue to expand the scope of epigenetic modifications in genomic elements that can be interrogated by genome-wide methods. Our findings using RRBS suggest that genomic loci outside of promoters and genic elements are associated with differential methylation in the context of exposure to maternal obesity (Fig 2). The role of intergenic DNA methylation modifications associated with environmental exposures is not well understood, but may regulate the activity of distant enhancers and the transcriptional repression of transposable elements [83, 84]. Previous findings using the 450K array, which has limited coverage of intergenic loci, have reported differential methylation at several intergenic regions in relation to BMI in the context of obesity [85, 86]. Our analysis of sequencing data obtained in this study suggest that differential methylation in intergenic regions may be a more prominent feature of DNA modifications associated with maternal obesity than previously thought, at least at CpG-dense loci, and this should be an important consideration for future research.
Our findings should be considered in light of study strengths and limitations, including small sample size. Strengths of this investigation include the use of two independent cohorts for comparison of differentially methylated genes. We acknowledge that the different platforms and associated analytical methods for each cohort (RRBS for the OBS samples, and 450K Illumina microarray for the ALSPAC cohort) are a limitation of this work. However, we note that both platforms are known to show a high correspondence for the detection of differential methylation in regulatory elements associated with genes, particularly when regional-based (DMR) analytical approaches are used [87]. In addition, samples in both cohorts were matched along dimensions known to influence DNA methylation signatures, including exclusions for gestational diabetes and preterm birth. This strategy avoided confounds of pregnancy complications shown to affect DNA methylation, but imposed limitations on the number of samples available. Thus, our findings should be considered preliminary. An additional limitation of this study concerns the use of whole blood, which may be less informative of metabolic dysregulation compared to muscle or fat. However, we note that blood constitutes a more readily accessible tissue, particularly in large-scale studies and studies in early life, and is known to reflect metabolic and immune pathways active in the context of obesity [88, 89]. Importantly, the hypothesis that genes associated with maternal obesity were differentially methylated at birth was supported in both cohorts. Additional work is needed to understand the potential relationship between these early methylation modifications detected at birth and later outcomes.
In animal studies, maternal obesity during pregnancy has been shown to result in a number of poor health indicators in offspring [90, 91]. Our results support the association of maternal obesity and biological signatures in humans at birth. There is a need to identify biomarkers prior to the emergence of poor health outcomes, when interventions that support mothers and their families are likely to be most effective. Ten per cent of school-aged children are estimated to be either overweight or obese worldwide [92]. These children have a significant likelihood of developing type 2 diabetes, heart disease and other co-morbidities before or during their early adulthood. Better prediction of vulnerable children and help to support optimal health will require a proven understanding of developmental mechanisms leading to adverse health outcomes.
In conclusion, maternal obesity is associated with DNA methylation modifications at loci in whole blood in a manner that is sex-specific. The present results support growing evidence indicating that sexual dimorphism is an important feature of the response to maternal obesity during early development. Our results support the feasibility of assessing sex-specific differences in DNA methylation in the very early post-partum period associated with the effect of maternal obesity. Features of DNA methylation modifications, including its relative stability in comparison to RNA and its potential as a mediator of adverse environmental exposures, have made them attractive candidates for the study of the Developmental Origins of Health and Disease (DOHaD). As there is accumulating evidence suggesting the mediating role of DNA methylation modifications in sex-specific childhood growth trajectories [93], the sites of DNA methylation modifications identified in this study may represent candidate loci for future studies including interventions targeting metabolic processes and diet. The identification of DNA methylation modifications in peripheral tissues such as blood should advance translational studies of the impact of maternal obesity on epigenetics and subsequent human disease.
Supporting information
X axis shows percent methylation per CpG site and y axis shows the frequency. C: Neonates from non-obese control mothers (n = 10/sex), O: Neonates from mothers with obesity (n = 10/sex).
(PDF)
C: neonates of non-obese control mothers (n = 10/sex), O: neonates of mothers with obesity (n = 10/sex).
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Dr. Hirotaka Hamada for critical reading of an earlier version of the manuscript and Dr. Wilfred C. de Vega for assistance in bioinformatics analyses, and Ms. Sheryl L Hewko for assistance with the REB and subject selection. OBS: The authors acknowledge the contribution and support of Ontario Birth Study Team members. In addition, we thank, and are extremely grateful to, all the women who took part in this study (www.ontariobirthstudy.com). ALSPAC: We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Data Availability
As the study data contain potentially identifying or sensitive patient information, datasets generated from OBS cohort are available from the OBS executive committee on reasonable request and in accordance with the OBS guidelines (ontariobirthstudy.MSH@sinaihealth.ca). ALSPAC data are available upon application approval by the executive committee (alspac-executive@bristol.ac.uk).
Funding Statement
AS was supported by the postdoctoral fellowship from Canadian Institutes of Health Research (CIHR). Supported by a Foundation grant from the Canadian Institutes of Health Research (FDN-148368) to SGM. Funding for the Ontario Birth Study has been provided by Mount Sinai Hospital, Mount Sinai Hospital Foundation, and the Lunenfeld-Tanenbaum Research Institute. ALSPAC: The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Drs. Briollais, Matthews and McGowan will serve as guarantor for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). This research was specifically funded by Wellcome Trust and MRC grant #076467/Z/05/Z, BBSRC grant BBI025751/1 and BB/I025263/1 (Dr. C. Relton), IEU grant codes MC_UU_12013/1 & MC_UU_12013/2 & MC_UU_12013/8 (Dr. G. Davey-Smith), National Institute of Child and Human grant R01HD068437 (Dr. T. Barker), NIH grant 5RO1AI121226-02 (Dr. H. Zhang), CONTAMED EU collaborative Project grant #212502 (Dr. A. Kortenkamp).
References
- 1.Afshin A, Reitsma MB, Murray CJL (2017) Health Effects of Overweight and Obesity in 195 Countries. N Engl J Med 377: 1496–1497. [Google Scholar]
- 2.Garn SM, Cole PE, Bailey SM (1979) Living together as a factor in family-line resemblances. Hum Biol 51: 565–587. [PubMed] [Google Scholar]
- 3.Rowe NH, Garn SM, Clark DC, Guire KE (1976) The effect of age, sex, race, and economic status on dental caries experience of the permanent dentition. Committee to Review the Ten-State Nutrition Survey of 1968–1970. Pediatrics 57: 457–461. [PubMed] [Google Scholar]
- 4.Whitaker RC (2004) Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114: e29–36. doi: 10.1542/peds.114.1.e29 [DOI] [PubMed] [Google Scholar]
- 5.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, et al. (2009) Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 90: 1303–1313. doi: 10.3945/ajcn.2008.27416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, et al. (2008) Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51: 383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477 [DOI] [PubMed] [Google Scholar]
- 7.Yokomizo H, Inoguchi T, Sonoda N, Sakaki Y, Maeda Y, et al. (2014) Maternal high-fat diet induces insulin resistance and deterioration of pancreatic beta-cell function in adult offspring with sex differences in mice. Am J Physiol Endocrinol Metab 306: E1163–1175. doi: 10.1152/ajpendo.00688.2013 [DOI] [PubMed] [Google Scholar]
- 8.Dearden L, Bouret SG, Ozanne SE (2018) Sex and gender differences in developmental programming of metabolism. Mol Metab 15: 8–19. doi: 10.1016/j.molmet.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauvais-Jarvis F (2015) Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of sex differences 6: 1–9. doi: 10.1186/s13293-014-0019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Power ML, Schulkin J (2008) Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. British Journal of Nutrition 99: 931–940. [Google Scholar]
- 11.Barker DJ (1990) The fetal and infant origins of adult disease. BMJ 301: 1111. doi: 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73. doi: 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharp GC, Lawlor DA, Richmond RC, Fraser A, Simpkin A, et al. (2015) Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 44: 1288–1304. doi: 10.1093/ije/dyv042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp GC, Salas LA, Monnereau C, Allard C, Yousefi P, et al. (2017) Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum Mol Genet 26: 4067–4085. doi: 10.1093/hmg/ddx290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soubry A, Murphy SK, Wang F, Huang Z, Vidal AC, et al. (2015) Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obes (Lond) 39: 650–657. doi: 10.1038/ijo.2013.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, et al. (2013) Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Med 11: 29. doi: 10.1186/1741-7015-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold AP, Gorski RA (1984) Gonadal steroid induction of structural sex differences in the central nervous system. Annual review of neuroscience 7: 413–442. doi: 10.1146/annurev.ne.07.030184.002213 [DOI] [PubMed] [Google Scholar]
- 18.Abramovich D (1974) Human sexual differentiation—in utero influences. BJOG: An International Journal of Obstetrics & Gynaecology 81: 448–453. doi: 10.1111/j.1471-0528.1974.tb00494.x [DOI] [PubMed] [Google Scholar]
- 19.Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, et al. (2012) The number of x chromosomes causes sex differences in adiposity in mice. PLoS genetics 8: e1002709. doi: 10.1371/journal.pgen.1002709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz LA, Kota SK, Feil R (2010) Genome-wide DNA demethylation in mammals. Genome biology 11: 1–4. doi: 10.1186/gb-2010-11-3-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Hajj N, Pliushch G, Schneider E, Dittrich M, Müller T, et al. (2013) Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes 62: 1320–1328. doi: 10.2337/db12-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finer S, Mathews C, Lowe R, Smart M, Hillman S, et al. (2015) Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Human molecular genetics 24: 3021–3029. doi: 10.1093/hmg/ddv013 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Hoyo C, Murphy S, Huang Z, Overcash F, et al. (2013) DNA methylation at imprint regulatory regions in preterm birth and infection. Am J Obstet Gynecol 208: 395 e391–397. doi: 10.1016/j.ajog.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki A, Eng ME, Lee AH, Kostaki A, Matthews SG (2021) DNA methylome signatures of prenatal exposure to synthetic glucocorticoids in hippocampus and peripheral whole blood of female guinea pigs in early life. Translational psychiatry 11: 1–9. doi: 10.1038/s41398-020-01158-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Consortium EP (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57. doi: 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bibikova M (2016) DNA Methylation Microarrays. Epigenomics in Health and Disease: Elsevier. pp. 19–46. [Google Scholar]
- 27.Anderson LN, Knight JA, Hung RJ, Hewko SL, Seeto RA, et al. (2018) The Ontario Birth Study: A prospective pregnancy cohort study integrating perinatal research into clinical care. Paediatr Perinat Epidemiol 32: 290–301. doi: 10.1111/ppe.12473 [DOI] [PubMed] [Google Scholar]
- 28.Sahota DS, Kagan KO, Lau TK, Leung TY, Nicolaides KH (2008) Customized birth weight: coefficients and validation of models in a UK population. Ultrasound Obstet Gynecol 32: 884–889. doi: 10.1002/uog.5372 [DOI] [PubMed] [Google Scholar]
- 29.Suter M, Ma J, Harris A, Patterson L, Brown KA, et al. (2011) Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics 6: 1284–1294. doi: 10.4161/epi.6.11.17819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghantous A, Saffery R, Cros MP, Ponsonby AL, Hirschfeld S, et al. (2014) Optimized DNA extraction from neonatal dried blood spots: application in methylome profiling. BMC Biotechnol 14: 60. doi: 10.1186/1472-6750-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki A, Kim B, Murphy KE, Matthews SG (2020) Impact of ex vivo sample handling on DNA methylation profiles in human cord blood and neonatal dried blood spots. Frontiers in Genetics 11: 224. doi: 10.3389/fgene.2020.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krueger F, Andrews SR (2011) Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27: 1571–1572. doi: 10.1093/bioinformatics/btr167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, et al. (2012) methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol 13: R87. doi: 10.1186/gb-2012-13-10-r87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziller MJ, Hansen KD, Meissner A, Aryee MJ (2015) Coverage recommendations for methylation analysis by whole-genome bisulfite sequencing. Nat Methods 12: 230–232, 231 p following 232. doi: 10.1038/nmeth.3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen KD, Langmead B, Irizarry RA (2012) BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol 13: R83. doi: 10.1186/gb-2012-13-10-r83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H-Q, Tuominen LK, Tsai C-J (2011) SLIM: a sliding linear model for estimating the proportion of true null hypotheses in datasets with dependence structures. Bioinformatics 27: 225–231. doi: 10.1093/bioinformatics/btq650 [DOI] [PubMed] [Google Scholar]
- 38.de Vega WC, Herrera S, Vernon SD, McGowan PO (2017) Epigenetic modifications and glucocorticoid sensitivity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). BMC Med Genomics 10: 11. doi: 10.1186/s12920-017-0248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott HR, Tillin T, McArdle WL, Ho K, Duggirala A, et al. (2014) Differences in smoking associated DNA methylation patterns in South Asians and Europeans. Clin Epigenetics 6: 4. doi: 10.1186/1868-7083-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Essex MJ, Boyce WT, Hertzman C, Lam LL, Armstrong JM, et al. (2013) Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Dev 84: 58–75. doi: 10.1111/j.1467-8624.2011.01641.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrera S, de Vega WC, Ashbrook D, Vernon SD, McGowan PO (2018) Genome-epigenome interactions associated with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Epigenetics 13: 1174–1190. doi: 10.1080/15592294.2018.1549769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Vega WC, Erdman L, Vernon SD, Goldenberg A, McGowan PO (2018) Integration of DNA methylation & health scores identifies subtypes in myalgic encephalomyelitis/chronic fatigue syndrome. Epigenomics 10: 539–557. doi: 10.2217/epi-2017-0150 [DOI] [PubMed] [Google Scholar]
- 43.Kishore K, de Pretis S, Lister R, Morelli MJ, Bianchi V, et al. (2015) methylPipe and compEpiTools: a suite of R packages for the integrative analysis of epigenomics data. BMC Bioinformatics 16: 313. doi: 10.1186/s12859-015-0742-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bock C (2012) Analysing and interpreting DNA methylation data. Nat Rev Genet 13: 705–719. doi: 10.1038/nrg3273 [DOI] [PubMed] [Google Scholar]
- 45.Ziller MJ, Gu H, Müller F, Donaghey J, Tsai LT-Y, et al. (2013) Charting a dynamic DNA methylation landscape of the human genome. Nature 500: 477–481. doi: 10.1038/nature12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, et al. (2013) Cohort profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology 42: 111–127. doi: 10.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, et al. (2013) Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International journal of epidemiology 42: 97–110. doi: 10.1093/ije/dys066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.http://www.bristol.ac.uk/alspac/researchers/our-data/. (Avon Longitudinal Study of Parents and Children [accessed Sept 1, 2020].).
- 49.Relton CL, Gaunt T, McArdle W, Ho K, Duggirala A, et al. (2015) Data resource profile: accessible resource for integrated epigenomic studies (ARIES). International journal of epidemiology 44: 1181–1190. doi: 10.1093/ije/dyv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma X, Wang Y-W, Zhang MQ, Gazdar AF (2013) DNA methylation data analysis and its application to cancer research. Epigenomics 5: 301–316. doi: 10.2217/epi.13.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, et al. (2014) Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30: 1363–1369. doi: 10.1093/bioinformatics/btu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K, et al. (2015) De novo identification of differentially methylated regions in the human genome. Epigenetics & chromatin 8: 1–16. doi: 10.1186/1756-8935-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satterthwaite FE (1946) An approximate distribution of estimates of variance components. Biometrics bulletin 2: 110–114. [PubMed] [Google Scholar]
- 54.Huang D, Sherman B, Lempicki R (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13. gkn923 [pii]; 10.1093/nar/gkn923 [doi]. doi: 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, et al. (2017) Influence of maternal obesity on the long-term health of offspring. The lancet Diabetes & endocrinology 5: 53–64. doi: 10.1016/S2213-8587(16)30107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moody L, Shao J, Chen H, Pan Y-X (2019) Maternal low-fat diet programs the hepatic epigenome despite exposure to an obesogenic postnatal diet. Nutrients 11: 2075. [Google Scholar]
- 57.Garg N, Thakur S, McMahan CA, Adamo ML (2011) High fat diet induced insulin resistance and glucose intolerance are gender-specific in IGF-1R heterozygous mice. Biochemical and biophysical research communications 413: 476–480. doi: 10.1016/j.bbrc.2011.08.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nikoshkov A, Sunkari VG, Savu O, Forsberg E, Catrina S-B, et al. (2011) Epigenetic DNA methylation in the promoters of the Igf1 receptor and insulin receptor genes in db/db mice. Epigenetics 6: 405–409. doi: 10.4161/epi.6.4.14791 [DOI] [PubMed] [Google Scholar]
- 59.Reik W, Constância M, Fowden A, Anderson N, Dean W, et al. (2003) Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. The Journal of physiology 547: 35–44. doi: 10.1113/jphysiol.2002.033274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butler MG (2011) Prader-Willi syndrome: obesity due to genomic imprinting. Current genomics 12: 204–215. doi: 10.2174/138920211795677877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen P, Piaggi P, Traurig M, Bogardus C, Knowler WC, et al. (2017) Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia 60: 645–655. doi: 10.1007/s00125-016-4203-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim IY, Lin X, Teh AL, Wu Y, Chen L, et al. (2022) Dichotomy in the Impact of Elevated Maternal Glucose Levels on Neonatal Epigenome. The Journal of Clinical Endocrinology & Metabolism 107: e1277–e1292. doi: 10.1210/clinem/dgab710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugiyama T, Benitez CM, Ghodasara A, Liu L, McLean GW, et al. (2013) Reconstituting pancreas development from purified progenitor cells reveals genes essential for islet differentiation. Proceedings of the National Academy of Sciences 110: 12691–12696. doi: 10.1073/pnas.1304507110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, et al. (2009) Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional complex. Nature 460: 1154–1158. doi: 10.1038/nature08262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, et al. (2011) Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of clinical investigation 121: 96–105. doi: 10.1172/JCI44271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benton MC, Johnstone A, Eccles D, Harmon B, Hayes MT, et al. (2015) An analysis of DNA methylation in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. Genome biology 16: 1–21. doi: 10.1186/s13059-014-0572-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serrano A, Asnani-Kishnani M, Couturier C, Astier J, Palou A, et al. (2020) DNA methylation changes are associated with the programming of white adipose tissue browning features by resveratrol and nicotinamide riboside neonatal supplementations in mice. Nutrients 12: 461. doi: 10.3390/nu12020461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsukasaki K, Miller CW, Greenspun E, Eshaghian S, Kawabata H, et al. (2001) Mutations in the mitotic check point gene, MAD1L1, in human cancers. Oncogene 20: 3301–3305. doi: 10.1038/sj.onc.1204421 [DOI] [PubMed] [Google Scholar]
- 69.Karsli-Ceppioglu S, Ngollo M, Adjakly M, Dagdemir A, Judes G, et al. (2015) Genome-wide DNA methylation modified by soy phytoestrogens: role for epigenetic therapeutics in prostate cancer? OMICS: A Journal of Integrative Biology 19: 209–219. doi: 10.1089/omi.2014.0142 [DOI] [PubMed] [Google Scholar]
- 70.Torii S, Kubota C, Saito N, Kawano A, Hou N, et al. (2018) The pseudophosphatase phogrin enables glucose-stimulated insulin signaling in pancreatic β cells. Journal of Biological Chemistry 293: 5920–5933. doi: 10.1074/jbc.RA117.000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lan MS, Wasserfall C, Maclaren NK, Notkins AL (1996) IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A 93: 6367–6370. doi: 10.1073/pnas.93.13.6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, et al. (1996) Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A 93: 2307–2311. doi: 10.1073/pnas.93.6.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wasmeier C, Hutton JC (1996) Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem 271: 18161–18170. doi: 10.1074/jbc.271.30.18161 [DOI] [PubMed] [Google Scholar]
- 74.Weng X, Liu F, Zhang H, Kan M, Wang T, et al. (2018) Genome-wide DNA methylation profiling in infants born to gestational diabetes mellitus. Diabetes Research and Clinical Practice 142: 10–18. doi: 10.1016/j.diabres.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 75.Awamleh Z, Butcher DT, Hanley A, Retnakaran R, Haertle L, et al. (2021) Exposure to Gestational Diabetes Mellitus (GDM) alters DNA methylation in placenta and fetal cord blood. Diabetes Research and Clinical Practice 174: 108690. doi: 10.1016/j.diabres.2021.108690 [DOI] [PubMed] [Google Scholar]
- 76.Yang I, Zhang W, Davidson E, Fingerlin T, Kechris K, et al. (2018) Epigenetic marks of in utero exposure to gestational diabetes and childhood adiposity outcomes: the EPOCH study. Diabetic Medicine 35: 612–620. doi: 10.1111/dme.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S (2019) The association of genetically controlled CpG methylation (cg158269415) of protein tyrosine phosphatase, receptor type N2 (PTPRN2) with childhood obesity. Scientific reports 9: 1–7. doi: 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishna RG, Vishnu Bhat B, Bobby Z, Papa D, Badhe B, et al. (2020) Identification of differentially methylated candidate genes and their biological significance in IUGR neonates by methylation EPIC array. The Journal of Maternal-Fetal & Neonatal Medicine: 1–9. doi: 10.1080/14767058.2020.1727881 [DOI] [PubMed] [Google Scholar]
- 79.Williams L, Seki Y, Delahaye F, Cheng A, Fuloria M, et al. (2016) DNA hypermethylation of CD3+ T cells from cord blood of infants exposed to intrauterine growth restriction. Diabetologia 59: 1714–1723. doi: 10.1007/s00125-016-3983-7 [DOI] [PubMed] [Google Scholar]
- 80.Li S, Wang W, Zhang D, Li W, Lund J, et al. (2021) Differential regulation of the DNA methylome in adults born during the Great Chinese Famine in 1959–1961. Genomics 113: 3907–3918. doi: 10.1016/j.ygeno.2021.09.018 [DOI] [PubMed] [Google Scholar]
- 81.Yang Z, Phung H, Hughes A-M, Sherwood S, Harper E, et al. (2019) Trends in overweight and obesity by socioeconomic status in Year 6 school children, Australian Capital Territory, 2006–2018. BMC Public Health 19: 1–10. doi: 10.1186/s12889-018-6343-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC (2018) Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 141. [Google Scholar]
- 83.Zhou W, Liang G, Molloy PL, Jones PA (2020) DNA methylation enables transposable element-driven genome expansion. Proceedings of the National Academy of Sciences 117: 19359–19366. doi: 10.1073/pnas.1921719117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, et al. (2006) DNA methylation profiling of human chromosomes 6, 20 and 22. Nature genetics 38: 1378–1385. doi: 10.1038/ng1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van den Dungen MW, Murk AJ, Kok DE, Steegenga WT (2016) Comprehensive DNA methylation and gene expression profiling in differentiating human adipocytes. Journal of cellular biochemistry 117: 2707–2718. doi: 10.1002/jcb.25568 [DOI] [PubMed] [Google Scholar]
- 86.Sayols-Baixeras S, Subirana I, Fernández-Sanlés A, Sentí M, Lluís-Ganella C, et al. (2017) DNA methylation and obesity traits: An epigenome-wide association study. The REGICOR study. Epigenetics 12: 909–916. doi: 10.1080/15592294.2017.1363951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carmona JJ, Accomando WP, Binder AM, Hutchinson JN, Pantano L, et al. (2017) Empirical comparison of reduced representation bisulfite sequencing and Infinium BeadChip reproducibility and coverage of DNA methylation in humans. NPJ genomic medicine 2: 1–10. doi: 10.1038/s41525-016-0002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Heredia FP, Gómez-Martínez S, Marcos A (2012) Obesity, inflammation and the immune system. Proceedings of the Nutrition Society 71: 332–338. doi: 10.1017/S0029665112000092 [DOI] [PubMed] [Google Scholar]
- 89.Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132: 2169–2180. doi: 10.1053/j.gastro.2007.03.059 [DOI] [PubMed] [Google Scholar]
- 90.Shrestha N, Ezechukwu HC, Holland OJ, Hryciw DH (2020) Developmental programming of peripheral diseases in offspring exposed to maternal obesity during pregnancy. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 319: R507–R516. doi: 10.1152/ajpregu.00214.2020 [DOI] [PubMed] [Google Scholar]
- 91.Nathanielsz PW, Poston L, Taylor PD (2007) In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Clinics in perinatology 34: 515–526. doi: 10.1016/j.clp.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 92.Lobstein T, Baur L, Uauy R, TaskForce IIO (2004) Obesity in children and young people: a crisis in public health. Obes Rev 5 Suppl 1: 4–104. doi: 10.1111/j.1467-789X.2004.00133.x [DOI] [PubMed] [Google Scholar]
- 93.Briollais L, Rustand D, Allard C, Wu Y, Xu J, et al. (2021) DNA methylation mediates the association between breastfeeding and early-life growth trajectories. Clinical epigenetics 13: 1–17. doi: 10.1186/s13148-020-00979-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
X axis shows percent methylation per CpG site and y axis shows the frequency. C: Neonates from non-obese control mothers (n = 10/sex), O: Neonates from mothers with obesity (n = 10/sex).
(PDF)
C: neonates of non-obese control mothers (n = 10/sex), O: neonates of mothers with obesity (n = 10/sex).
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
As the study data contain potentially identifying or sensitive patient information, datasets generated from OBS cohort are available from the OBS executive committee on reasonable request and in accordance with the OBS guidelines (ontariobirthstudy.MSH@sinaihealth.ca). ALSPAC data are available upon application approval by the executive committee (alspac-executive@bristol.ac.uk).