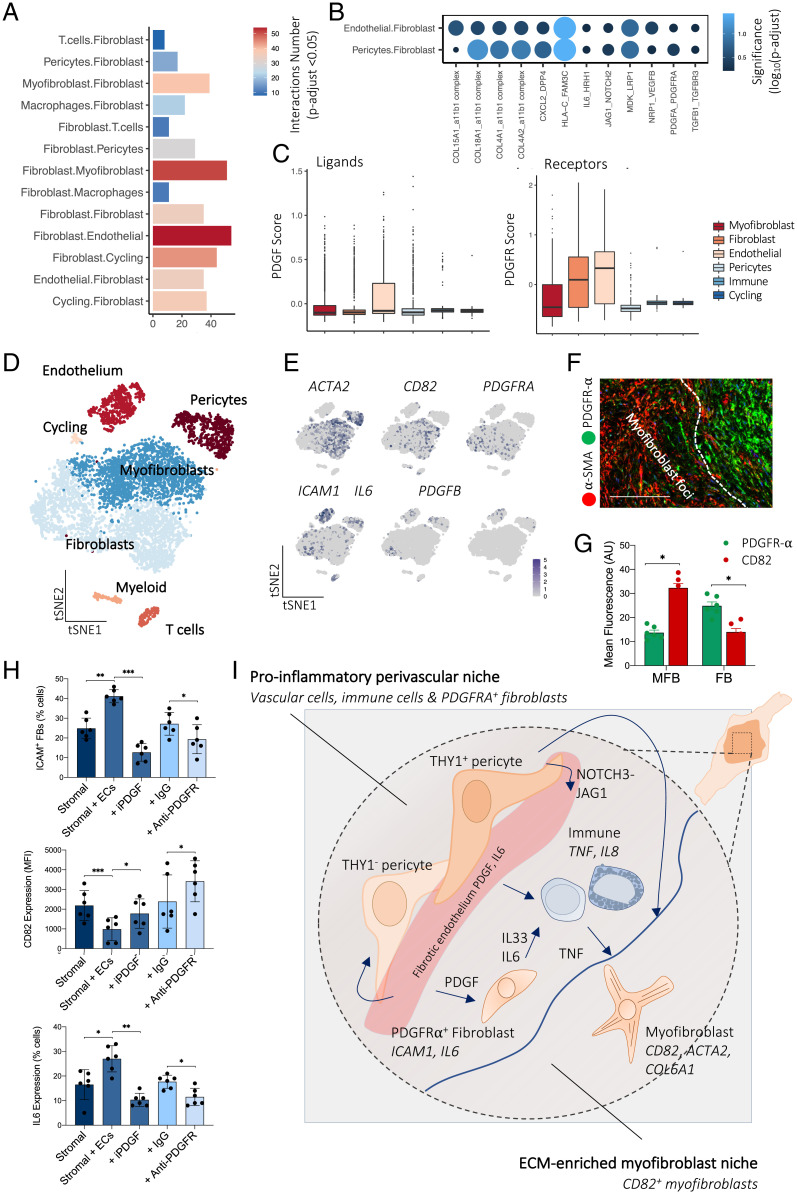
Fig. 4.
Endothelial cells sustain fibroblast phenotype. (A) Barplot showing the number of significant interactions between cell types in DD nodules defined by CellPhoneDB analysis of scRNA-seq (n = 12 DD patients). Adjusted P < 0.05 was defined as significant (two-sided Wilcoxon rank-sum test, BH FDR correction). (B) Dotplot showing significance (color and size) of receptor–ligand pairs in significantly enriched pathways following interaction analysis of scRNA-seq. Two-sided Wilcoxon rank-sum test, BH FDR correction. (C) Box and whisker plots showing PDGF ligand and receptor gene module expression in DD cell types defined in scRNA-seq (n = 12 DD patients). (D) tSNE projection of scRNA-seq in entire DD nodules showing major cell types (n = 12 DD patients). (E) tSNE projections of scRNA-seq showing RNA expression (log(UMI + 1)) in DD cells of selected genes characterizing major cell populations. (F) Confocal images of immunofluorescence showing PDFGR-α expression in fibroblasts and CD82 in myofibroblasts within DD nodules (n = 3 DD patients). (Scale bar, 40 µm.) (G) Barplot showing quantitative analysis (mean fluorescence; arbitrary unit, AU) of immunofluorescence microscopy of PDFGR-α and CD82 expression in DD nodules. MFB represents myofibroblast foci and FB represents the fibroblast niche in DD nodules, two-sided unpaired t test, mean ± SEM (n = 6 DD patients). *P < 0.05. (H) Barplots of flow cytometry analysis of DD and endothelial cell coculture assay. iPDGF represents crenolanib and anti-PDGFR represents the anti–PDGFR-α/β antibody mixture. Two-sided unpaired t test, mean ± SEM (n = 6 DD patients). *P < 0.05; **P < 0.01; ***P < 0.001. (I) Schematic illustrating stromal–immune cell cross-talk in DD. Immune cells (mast cells and M2 macrophages) are the predominant source of TNF that leads to the development of the myofibroblast phenotype. Myofibroblasts are a significant source of IL33, which further activates TNF secretion from immune cells. The endothelium acts to promote formation of fibroblasts.