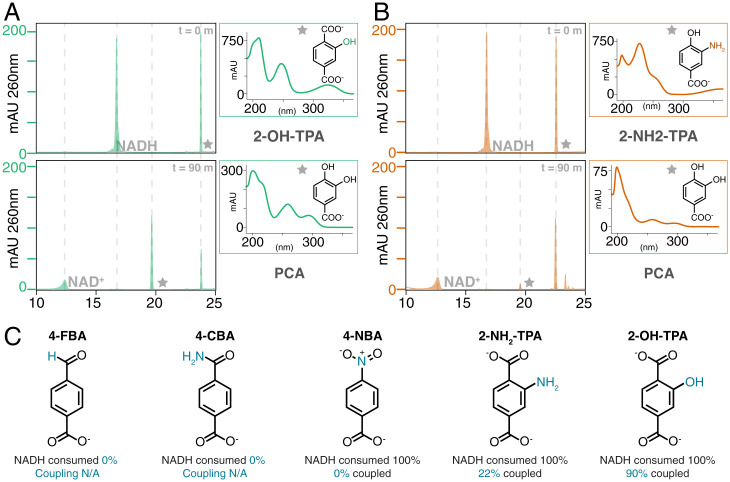
Fig. 3.
TPADO permits only structurally conservative substitutions on TPA analogs. Consumption of NADH and substrate analog following incubation in air with the TPADO/reductase system was measured via HPLC. (A and B) HPLC chromatograms, in milliabsorbance units (mAU) versus retention time, were measured at the outset (Upper) and 90-min end point (Lower) of the reaction with 2-OH-TPA (A) and 2-NH2-TPA (B). Stars indicate the peaks correlating to the adjacent UV/vis callout. Retention time and UV/vis spectra of the products for both the 2-OH-TPA and 2-NH2-TPA reactions match those of PCA, although the latter achieved substantially lower coupling. The observed PCA product for both 2-substituted TPA analogs definitively indicates 1,2-dioxygenation. (C) Analogs 4-FBA and 4-CBA showed no NADH consumption, while 4-NBA showed 100% consumption of NADH with no aromatic product formation (0% coupled). 2-NH2-TPA and 2-OH-TPA showed 22 and 100% coupling, respectively.